Behavioral Characteristics Serpophaginine Tyrannids
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
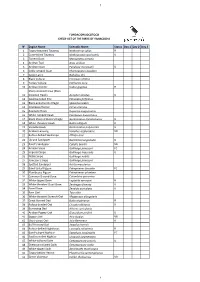
N° English Name Scientific Name Status Day 1
1 FUNDACIÓN JOCOTOCO CHECK-LIST OF THE BIRDS OF YANACOCHA N° English Name Scientific Name Status Day 1 Day 2 Day 3 1 Tawny-breasted Tinamou Nothocercus julius R 2 Curve-billed Tinamou Nothoprocta curvirostris U 3 Torrent Duck Merganetta armata 4 Andean Teal Anas andium 5 Andean Guan Penelope montagnii U 6 Sickle-winged Guan Chamaepetes goudotii 7 Cattle Egret Bubulcus ibis 8 Black Vulture Coragyps atratus 9 Turkey Vulture Cathartes aura 10 Andean Condor Vultur gryphus R Sharp-shinned Hawk (Plain- 11 breasted Hawk) Accipiter striatus U 12 Swallow-tailed Kite Elanoides forficatus 13 Black-and-chestnut Eagle Spizaetus isidori 14 Cinereous Harrier Circus cinereus 15 Roadside Hawk Rupornis magnirostris 16 White-rumped Hawk Parabuteo leucorrhous 17 Black-chested Buzzard-Eagle Geranoaetus melanoleucus U 18 White-throated Hawk Buteo albigula R 19 Variable Hawk Geranoaetus polyosoma U 20 Andean Lapwing Vanellus resplendens VR 21 Rufous-bellied Seedsnipe Attagis gayi 22 Upland Sandpiper Bartramia longicauda R 23 Baird's Sandpiper Calidris bairdii VR 24 Andean Snipe Gallinago jamesoni FC 25 Imperial Snipe Gallinago imperialis U 26 Noble Snipe Gallinago nobilis 27 Jameson's Snipe Gallinago jamesoni 28 Spotted Sandpiper Actitis macularius 29 Band-tailed Pigeon Patagoienas fasciata FC 30 Plumbeous Pigeon Patagioenas plumbea 31 Common Ground-Dove Columbina passerina 32 White-tipped Dove Leptotila verreauxi R 33 White-throated Quail-Dove Zentrygon frenata U 34 Eared Dove Zenaida auriculata U 35 Barn Owl Tyto alba 36 White-throated Screech-Owl Megascops -

House Sparrow Eradication Attempt on Robinson Crusoe Island, Juan Fernández Archipelago, Chile
E. Hagen, J. Bonham and K. Campbell Hagen, E.; J. Bonham and K. Campbell. House sparrow eradication attempt on Robinson Crusoe Island, Juan Fernández Archipelago, Chile House sparrow eradication attempt on Robinson Crusoe Island, Juan Fernández Archipelago, Chile E. Hagen1, J. Bonham1 and K. Campbell1,2 1Island Conservation, Las Urbinas 53 Santiago, Chile. <[email protected]>.2School of Geography, Planning & Environmental Management, The University of Queensland, St Lucia 4072, Australia. Abstract House sparrows (Passer domesticus) compete with native bird species, consume crops, and are vectors for diseases in areas where they have been introduced. Sparrow eradication attempts aimed at eliminating these negative eff ects highlight the importance of deploying multiple alternative methods to remove individuals while maintaining the remaining population naïve to techniques. House sparrow eradication was attempted from Robinson Crusoe Island, Chile, in the austral winter of 2012 using an experimental approach sequencing passive multi-catch traps, passive single- catch traps, and then active multi-catch methods, and fi nally active single-catch methods. In parallel, multiple detection methods were employed and local stakeholders were engaged. The majority of removals were via passive trapping, and individuals were successfully targeted with active methods (mist nets and shooting). Automated acoustic recording, point counts and camera traps declined in power to detect individual sparrows as the population size decreased; however, we continued to detect sparrows at all population densities using visual observations, underscoring the importance of local residents’ participation in monitoring. Four surviving sparrows were known to persist at the conclusion of eff orts in 2012. Given the lack of formal biosecurity measures within the Juan Fernández archipelago, reinvasion is possible. -

Neotropical Birdingbirding T H E B I R D I N G M a G a Z I N E O F T H E Neotropical B I R D C L U B
NeotropicalNeotropical BirdingBirding THE BIRDING MAGAZINE OF THE NEOTROPICAL BIRD CLUB Number 28 • Spring 2021 Neotropical Birding BIRDING MAGAZINE OF THE NEOTROPICAL BIRD CLUB • NUMBER 28 • SPRING 2021 EDITORIAL PHOTOSPOT 2 Welcome to Neotropical Birding 28 69 Photospot: Ocellated Crake in the JAMES LOWEN Brazilian Cerrado DANIEL BRANCH BIRDING SITES REVIEWS 3 Uruguay: gateway to Neotropical birdlife 72 Birds of Argentina and the ADRIÁN B. AZPIROZ South-west Atlantic RAYMOND JEFFERS FEATURE 73 The birds of Cuba: an annotated Saving Galápagos landbirds checklist 17 BEN STOCKWELL PETE MORRIS NEW BOOK 74 Illustrated checklist of the mammals of the world 28 Field identification of some look-alike JAMES LOWEN Serpophaga tyrannulets and Plain Inezia from Argentina All the birds of the world MARK PEARMAN AND JUAN I. ARETA 75 JAMES LOWEN FEATURE Aves do Sudeste do Brasil 77 JAMES LOWEN 35 Conservation action does prevent extinctions: a Neotropical perspective NBC NOTICEBOARD JAMES LOWEN NBC Noticeboard IDENTIFICATION WORKSHOP 78 CHRIS BALCHIN 45 The identification of Junin Grebe CONSERVATION FUND Podiceps taczanowskii DAVID FISHER NBC Conservation Awards, 2019–20 80 ROB CLAY FEATURE FRONT COVER 49 Global Bird Weekend – in the Neotropics Singing male Chestnut Seedeater PENNY ROBINSON Sporophila cinnamomea, west of Guichón, Paysandú, Uruguay, FEATURE November 2009 (Adrián Azpiroz/Wild Punta del Este). One of several globally 53 Saving the lord of the Andean skies threatened birds that are readily JAMES LOWEN found in Uruguay’s native grasslands (p3). This capuchino also breeds in SPLITS, LUMPS AND SHUFFLES neighbouring Argentina, a country that features twice in this issue (p28, p72). Splits, lumps and shuffles 60 THOMAS S. -

Listas De Aves 2019
List of bird of CUSCO BIRDING - CUSCO 3399 m.a.s.l N° ENGLISH NAME NOMBRE CIENTIFICO 1 Alder Flycatcher Empidonax alnorum 2 American Golden-Plover Pluvialis dominica 3 American Kestrel Falco sparverius 4 Amethyst-throated Sunangel Heliangelus amethysticollis 5 Andean Avocet Recurvirostra andina 6 Andean Cock-of-the-rock Rupicola peruvianus 7 Andean Condor Vultur gryphus 8 Andean Duck Oxyura ferruginea 9 Andean Flicker Colaptes rupicola 10 Andean Goose Oressochen melanopterus 11 Andean Guan Penelope montagnii 12 Andean Gull Chroicocephalus serranus 13 Andean Hillstar Oreotrochilus estella 14 Andean Lapwing Vanellus resplendens 15 Andean Motmot Momotus aequatorialis 16 Andean Negrito Lessonia oreas 17 Andean Parakeet Bolborhynchus orbygnesius 18 Andean Solitaire Myadestes ralloides 19 Andean Swallow Orochelidon andecola 20 Andean Swift Aeronautes andecolus 21 Andean Tinamou Nothoprocta pentlandii 22 Andean Tit-Spinetail Leptasthenura andicola 23 Aplomado Falcon Falco femoralis 24 Ash-breasted Sierra-Finch Phrygilus plebejus 25 Ash-breasted Tit-Tyrant Anairetes alpinus 26 Ashy-headed Tyrannulet Phyllomyias cinereiceps 27 Azara's Spinetail Synallaxis azarae 28 Baird's Sandpiper Calidris bairdii 29 Bananaquit Coereba flaveola 30 Band-tailed Fruiteater Pipreola intermedia 31 Band-tailed Pigeon Patagioenas fasciata 32 Band-tailed Seedeater Catamenia analis 33 Band-tailed Sierra-Finch Phrygilus alaudinus 34 Band-winged Nightjar Systellura longirostris 35 Bank Swallow Riparia riparia 36 Bar-bellied Woodpecker Veniliornis nigriceps 37 Bare-faced -

UNIVERSIDAD COMPLUTENSE DE MADRID FACULTAD DE CIENCIAS BIOLÓGICAS Departamento De Zoología Y Antropología Física
UNIVERSIDAD COMPLUTENSE DE MADRID FACULTAD DE CIENCIAS BIOLÓGICAS Departamento de Zoología y Antropología Física TESIS DOCTORAL Diversidad y especificidad de simbiontes en aves neotropicales Diversity and host specificity of symbionts in neotropical birds MEMORIA PARA OPTAR AL GRADO DE DOCTOR PRESENTADA POR Michaël André Jean Moens Directores Javier Pérez Tris Laura Benítez Rico Madrid, 2017 © Michaël André Jean Moens, 2016 Diversidad y Especificidad de Simbiontes en Aves Neotropicales (Diversity and Host Specificity of Symbionts in Neotropical birds.) Tesis doctoral de: Michaël André Jean Moens Directores : Javier Pérez Tris Laura Benítez Rico Madrid, 2016 © Michaël André Jean Moens, 2016 Cover Front Chestnut-breasted Coronet (Boissoneaua matthewsii) Taken at the San Isidro Reserve, Ecuador. Copyright © Jaime Culebras Cover Back Royal Flycatcher (Onychorhynchus coronatus) Taken at the Nouragues Reserve, French Guiana. Copyright © Borja Milá UNIVERSIDAD COMPLUTENSE DE MADRID FACULTAD DE CIENCIAS BIOLÓGICAS DEPARTAMENTO DE ZOOLOGÍA Y ANTROPOLOGÍA FÍSICA Diversidad y Especificidad de Simbiontes en Aves Neotropicales (Diversity and Host Specificity of Symbionts in Neotropical birds.) Tesis doctoral de: Michaël André Jean Moens Directores: Javier Pérez Tris Laura Benítez Rico Madrid, 2016 © Michaël André Jean Moens, 2016 UNIVERSIDAD COMPLUTENSE DE MADRID FACULTAD DE CIENCIAS BIOLÓGICAS DEPARTAMENTO DE ZOOLOGÍA Y ANTROPOLOGÍA FÍSICA Diversidad y Especificidad de Simbiontes en Aves Neotropicales (Diversity and Host Specificity of Symbionts -

Composition and Structure of Bird Flocks in a Temperate Forest of Central Chile
Revista Chilena de Ornitología 26(1): 33-36 Unión de Ornitólogos de Chile 2020 COMUNICACIÓN BREVE 33 COMPOSITION AND STRUCTURE OF BIRD FLOCKS IN A TEMPERATE FOREST OF CENTRAL CHILE Composición y estructura de bandadas en el bosque templado de Chile central FERNANDO MEDRANO1,2, MARÍA A. VUKASOVIC3,4, ROMINA CHIAPPE3,4 & CRISTIÁN F. ESTADES3,4 1Departamento de Biología Evolutiva, Ecologia i Ciències Ambientals, Institut de Recerca de la Biodiversitat (IRBio), Facultat de Biología, Universitat de Barcelona, Barcelona 08028, España. 2Red de Observadores de Aves y Vida Silvestre de Chile. Santiago, Chile. 3Unión de Ornitólogos de Chile (AvesChile). Santiago, Chile. 4Laboratorio de Ecología de Vida Silvestre. Facultad de Ciencias Forestales y Conservación de la Naturaleza. Santa Rosa #11315. La Pintana, Santiago, Chile. Correspondencia: F. Medrano, [email protected] RESUMEN.- Formar bandadas es una adaptación de las aves para aumentar la eficiencia de forrajeo y disminuir la depredación. Pese a que en bosques tropicales existen numerosos estudios sobre la formación de bandadas, en bosques templados de Sudamérica esta conducta ha sido escasamente documentada. En este estudio describi- mos la composición de 77 bandadas encontradas en un paisaje forestal de la región del Maule entre 1999-2002, y analizamos si existen relaciones entre las abundancias dentro de las bandadas entre especies. Usando simu- laciones de Monte Carlo analizamos la prevalencia de diferentes especies a formar bandadas y relacionamos la abundancia de las especies entre sí dentro de las bandadas. Cada bandada tuvo en promedio 10,5 individuos y la especie más frecuente fue Aphrastura spinicauda. Pese a que se ha señalado a A. -

Disentangling Leucocytozoon Parasite Diversity in the Neotropics Descriptions of Two New Species and Shortcomings of Molecular
IJP: Parasites and Wildlife 9 (2019) 159–173 Contents lists available at ScienceDirect IJP: Parasites and Wildlife journal homepage: www.elsevier.com/locate/ijppaw Disentangling Leucocytozoon parasite diversity in the neotropics: T Descriptions of two new species and shortcomings of molecular diagnostics for leucocytozoids ∗ Ingrid A. Lottaa, , Gediminas Valkiūnasb, M. Andreína Pachecoc, Ananías A. Escalantec, ∗∗ Sandra Rocío Hernándeza,d, Nubia E. Mattaa, a Universidad Nacional de Colombia - Sede Bogotá- Facultad de Ciencias - Departamento de Biología - Grupo de Investigación Caracterización Genética e Inmunología, Carrera 30 No. 45-03, Bogotá, 111321, Colombia b Institute of Ecology, Nature Research Centre, Akademijos 2, Vilnius-21, LT, 08412, Lithuania c Institute for Genomics and Evolutionary Medicine (iGEM), Temple University, Philadelphia, PA, USA d Red de Biología y Conservación de Vertebrados, Instituto de Ecología A.C., Xalapa, Veracruz, Mexico ARTICLE INFO ABSTRACT Keywords: Avian communities from South America harbor an extraordinary diversity of Leucocytozoon species Andean mountains (Haemosporida, Leucocytozoidae). Here, of 890 birds sampled, 10 (1.2%) were infected with Leucocytozoon Birds parasites. Among them, two new species were discovered and described. Leucocytozoon grallariae sp. nov. and Co-infection Leucocytozoon neotropicalis sp. nov. were found in non-migratory highland passeriforms belonging to the New species Grallaridae and Cotingidae, respectively. They both possess gametocytes in fusiform host cells. However, due to Cotingidae combining microscopic examination and molecular detection, it was revealed that these parasites were present Grallaridae Passeriforms in co-infections with other Leucocytozoon species, which gametocytes develop in roundish host cells, therefore exhibiting two highly distant parasite lineages isolated from the same samples. Remarkably, the lineages ob- tained by cloning the mtDNA genomes were not captured by the classic nested PCR, which amplifies a short fragment of cytochrome b gene. -

MORPHOLOGICAL and ECOLOGICAL EVOLUTION in OLD and NEW WORLD FLYCATCHERS a Dissertation Presented to the Faculty of the College O
MORPHOLOGICAL AND ECOLOGICAL EVOLUTION IN OLD AND NEW WORLD FLYCATCHERS A dissertation presented to the faculty of the College of Arts and Sciences of Ohio University In partial fulfillment of the requirements for the degree Doctor of Philosophy Clay E. Corbin August 2002 This dissertation entitled MORPHOLOGICAL AND ECOLOGICAL EVOLUTION IN OLD AND NEW WORLD FLYCATCHERS BY CLAY E. CORBIN has been approved for the Department of Biological Sciences and the College of Arts and Sciences by Donald B. Miles Associate Professor, Department of Biological Sciences Leslie A. Flemming Dean, College of Arts and Sciences CORBIN, C. E. Ph.D. August 2002. Biological Sciences. Morphological and Ecological Evolution in Old and New World Flycatchers (215pp.) Director of Dissertation: Donald B. Miles In both the Old and New Worlds, independent clades of sit-and-wait insectivorous birds have evolved. These independent radiations provide an excellent opportunity to test for convergent relationships between morphology and ecology at different ecological and phylogenetic levels. First, I test whether there is a significant adaptive relationship between ecology and morphology in North American and Southern African flycatcher communities. Second, using morphological traits and observations on foraging behavior, I test whether ecomorphological relationships are dependent upon locality. Third, using multivariate discrimination and cluster analysis on a morphological data set of five flycatcher clades, I address whether there is broad scale ecomorphological convergence among flycatcher clades and if morphology predicts a course measure of habitat preference. Finally, I test whether there is a common morphological axis of diversification and whether relative age of origin corresponds to the morphological variation exhibited by elaenia and tody-tyrant lineages. -

On the Origin and Evolution of Nest Building by Passerine Birds’
T H E C 0 N D 0 R r : : ,‘ “; i‘ . .. \ :i A JOURNAL OF AVIAN BIOLOGY ,I : Volume 99 Number 2 ’ I _ pg$$ij ,- The Condor 99~253-270 D The Cooper Ornithological Society 1997 ON THE ORIGIN AND EVOLUTION OF NEST BUILDING BY PASSERINE BIRDS’ NICHOLAS E. COLLIAS Departmentof Biology, Universityof California, Los Angeles, CA 90024-1606 Abstract. The object of this review is to relate nest-buildingbehavior to the origin and early evolution of passerinebirds (Order Passeriformes).I present evidence for the hypoth- esis that the combinationof small body size and the ability to place a constructednest where the bird chooses,helped make possiblea vast amountof adaptiveradiation. A great diversity of potential habitats especially accessibleto small birds was created in the late Tertiary by global climatic changes and by the continuing great evolutionary expansion of flowering plants and insects.Cavity or hole nests(in ground or tree), open-cupnests (outside of holes), and domed nests (with a constructedroof) were all present very early in evolution of the Passeriformes,as indicated by the presenceof all three of these basic nest types among the most primitive families of living passerinebirds. Secondary specializationsof these basic nest types are illustratedin the largest and most successfulfamilies of suboscinebirds. Nest site and nest form and structureoften help characterizethe genus, as is exemplified in the suboscinesby the ovenbirds(Furnariidae), a large family that builds among the most diverse nests of any family of birds. The domed nest is much more common among passerinesthan in non-passerines,and it is especially frequent among the very smallestpasserine birds the world over. -

Peru: Manu Biosphere Reserve September 3–18, 2019
PERU: MANU BIOSPHERE RESERVE SEPTEMBER 3–18, 2019 Red-and-green Macaw. Ara chloropterus. Photo: D. Ascanio. LEADERS: DAVID ASCANIO & PERCY AVENDAÑO LIST COMPILED BY: DAVID ASCANIO VICTOR EMANUEL NATURE TOURS, INC. 2525 WALLINGWOOD DRIVE, SUITE 1003 AUSTIN, TEXAS 78746 WWW.VENTBIRD.COM PERU: MANU BIOSPHERE RESERVE SEPTEMBER 3–18, 2019 By David Ascanio Photo album: https://flic.kr/s/aHsmHnbkJg When I started writing the Field Report for this amazing tour, I was on my flight back home. And, as I was enjoying the view from the plane’s window, I was wondering how to start an introductory paragraph highlighting the best experiences and birds of our Manu Biosphere Reserve tour. I found it to be a difficult task, not only because we came across an impressive number of habitats, but also because we saw so many wonderful birds! As I was still on that international flight, it didn’t take me long to figure out that I should, instead, divide this Field Report into four major areas and describe the amazing experiences and wonderful birds we enjoyed in each one. With that in mind, here we go! THE HIGH ANDES – PUNA Once we landed in Cusco, and after a wonderful breakfast, our tour started in the Huarcapay Lagoon, where 3 individuals of the rarely encountered Chilean Flamingo were observed. Here, we also saw Puna, Yellow-billed and Cinnamon teals, Spot-winged Pigeon, a vocal Plumbeous Rail, the beautiful White-tufted Grebe, several Andean Gulls, the secretive Rusty-fronted Canastero (endemic to Peru), and the Rufous-naped Ground-Tyrant, which is easily camouflaged by its gravel color. -

Bird Conservation International Molecular Characterisation of Haemoparasites in Forest Birds from Robinson Crusoe Island: Is
Bird Conservation International http://journals.cambridge.org/BCI Additional services for Bird Conservation International: Email alerts: Click here Subscriptions: Click here Commercial reprints: Click here Terms of use : Click here Molecular characterisation of haemoparasites in forest birds from Robinson Crusoe Island: Is the Austral Thrush a potential threat to endemic birds? JAVIER MARTÍNEZ, RODRIGO A. VÁSQUEZ, CRISTOBAL VENEGAS and SANTIAGO MERINO Bird Conservation International / Volume 25 / Issue 02 / June 2015, pp 139 - 152 DOI: 10.1017/S0959270914000227, Published online: 19 August 2014 Link to this article: http://journals.cambridge.org/abstract_S0959270914000227 How to cite this article: JAVIER MARTÍNEZ, RODRIGO A. VÁSQUEZ, CRISTOBAL VENEGAS and SANTIAGO MERINO (2015). Molecular characterisation of haemoparasites in forest birds from Robinson Crusoe Island: Is the Austral Thrush a potential threat to endemic birds?. Bird Conservation International, 25, pp 139-152 doi:10.1017/S0959270914000227 Request Permissions : Click here Downloaded from http://journals.cambridge.org/BCI, IP address: 212.128.9.27 on 22 May 2015 Bird Conservation International (2015) 25 :139 –152 . © BirdLife International, 2014 doi:10.1017/S0959270914000227 Molecular characterisation of haemoparasites in forest birds from Robinson Crusoe Island: Is the Austral Thrush a potential threat to endemic birds? JAVIER MARTÍNEZ , RODRIGO A. VÁSQUEZ , CRISTOBAL VENEGAS and SANTIAGO MERINO Summary The Juan Fernández Firecrown Sephanoides fernandensis and Juan Fernández Tit-Tyrant Anairetes fernandezianus are two endemic forest birds inhabiting Robinson Crusoe Island and are classified as ‘Critically Endangered’ and ‘Near Threatened’ respectively by IUCN. Previous research concluded that the two main factors involved in the decline of these birds were habitat degradation and the introduction of predator / competitor species. -

Gayanazoo 77(1) 2013.Indd
Gayana 77(1): 1-9, 2013. ISSN 0717-652X First description of the micro-habitat selection pattern of the island endemic Juan Fernandez Tit-tyrant Primera descripción del patrón de selección de micro-hábitat del Cachudito de Juan Fernández GERARDO E SOTO1,*, PABLO M VERGARA1, INGO J HAHN3, CHRISTIAN G PÉREZ-HERNÁNDEZ1, MARLENE E LIZAMA1, JULIA BAUMEISTER3 & JAIME PIZARRO2 1Departamento de Gestión Agraria, Universidad de Santiago de Chile, Alameda Libertador Bernardo O’Higgins 3363, Estación Central, Santiago, Región Metropolitana, Chile. * E-mail: [email protected] 2Departamento de Ingeniería Geográfi ca, Universidad de Santiago de Chile, Alameda Libertador Bernardo O’Higgins 3363, Estación Central, Santiago, Región Metropolitana, Chile. 3Institute of Landscape Ecology, University of Münster, Robert-Koch-Str. 28, D-48149 Münster, Germany RESUMEN Para las aves que son endémicas de islas oceánicas el nivel de especialización en el uso del hábitat puede ser un factor importante de considerar en el incremento del riesgo de extinción de estas especies. En este estudio, usamos datos de redes de niebla y radiotelemetría para determinar el patrón de uso de micro-hábitat por el Cachudito de Juan Fernández (Anairetes fernandezianus), una especie de ave endémica del archipiélago de Juan Fernández (Chile). El individuo seguido por radiotelemetría estableció su ámbito de hogar exclusivamente en áreas centrales del bosque nativo residual. Los modelos regresivos de abundancia y las Funciones de Utilización de Recursos mostraron fuertes preferencias de micro-hábitat por los cachuditos. Los valores de abundancia y utilización de recurso por los cachuditos decrecieron con la distancia al matorral exótico y aumentaron con la distancia a los claros hechos por humanos dentro bosque.