The Association Between Chiari Malformation Type I, Spinal Syrinx, and Scoliosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Craniosynostosis and Related Disorders Mark S
CLINICAL REPORT Guidance for the Clinician in Rendering Pediatric Care Identifying the Misshapen Head: Craniosynostosis and Related Disorders Mark S. Dias, MD, FAAP, FAANS,a Thomas Samson, MD, FAAP,b Elias B. Rizk, MD, FAAP, FAANS,a Lance S. Governale, MD, FAAP, FAANS,c Joan T. Richtsmeier, PhD,d SECTION ON NEUROLOGIC SURGERY, SECTION ON PLASTIC AND RECONSTRUCTIVE SURGERY Pediatric care providers, pediatricians, pediatric subspecialty physicians, and abstract other health care providers should be able to recognize children with abnormal head shapes that occur as a result of both synostotic and aSection of Pediatric Neurosurgery, Department of Neurosurgery and deformational processes. The purpose of this clinical report is to review the bDivision of Plastic Surgery, Department of Surgery, College of characteristic head shape changes, as well as secondary craniofacial Medicine and dDepartment of Anthropology, College of the Liberal Arts characteristics, that occur in the setting of the various primary and Huck Institutes of the Life Sciences, Pennsylvania State University, State College, Pennsylvania; and cLillian S. Wells Department of craniosynostoses and deformations. As an introduction, the physiology and Neurosurgery, College of Medicine, University of Florida, Gainesville, genetics of skull growth as well as the pathophysiology underlying Florida craniosynostosis are reviewed. This is followed by a description of each type of Clinical reports from the American Academy of Pediatrics benefit from primary craniosynostosis (metopic, unicoronal, bicoronal, sagittal, lambdoid, expertise and resources of liaisons and internal (AAP) and external reviewers. However, clinical reports from the American Academy of and frontosphenoidal) and their resultant head shape changes, with an Pediatrics may not reflect the views of the liaisons or the emphasis on differentiating conditions that require surgical correction from organizations or government agencies that they represent. -

Blueprint Genetics Craniosynostosis Panel
Craniosynostosis Panel Test code: MA2901 Is a 38 gene panel that includes assessment of non-coding variants. Is ideal for patients with craniosynostosis. About Craniosynostosis Craniosynostosis is defined as the premature fusion of one or more cranial sutures leading to secondary distortion of skull shape. It may result from a primary defect of ossification (primary craniosynostosis) or, more commonly, from a failure of brain growth (secondary craniosynostosis). Premature closure of the sutures (fibrous joints) causes the pressure inside of the head to increase and the skull or facial bones to change from a normal, symmetrical appearance resulting in skull deformities with a variable presentation. Craniosynostosis may occur in an isolated setting or as part of a syndrome with a variety of inheritance patterns and reccurrence risks. Craniosynostosis occurs in 1/2,200 live births. Availability 4 weeks Gene Set Description Genes in the Craniosynostosis Panel and their clinical significance Gene Associated phenotypes Inheritance ClinVar HGMD ALPL Odontohypophosphatasia, Hypophosphatasia perinatal lethal, AD/AR 78 291 infantile, juvenile and adult forms ALX3 Frontonasal dysplasia type 1 AR 8 8 ALX4 Frontonasal dysplasia type 2, Parietal foramina AD/AR 15 24 BMP4 Microphthalmia, syndromic, Orofacial cleft AD 8 39 CDC45 Meier-Gorlin syndrome 7 AR 10 19 EDNRB Hirschsprung disease, ABCD syndrome, Waardenburg syndrome AD/AR 12 66 EFNB1 Craniofrontonasal dysplasia XL 28 116 ERF Craniosynostosis 4 AD 17 16 ESCO2 SC phocomelia syndrome, Roberts syndrome -

Accelerating Matchmaking of Novel Dysmorphology Syndromes Through Clinical and Genomic Characterization of a Large Cohort
ORIGINAL RESEARCH ARTICLE © American College of Medical Genetics and Genomics Accelerating matchmaking of novel dysmorphology syndromes through clinical and genomic characterization of a large cohort Ranad Shaheen, PhD1, Nisha Patel, PhD1, Hanan Shamseldin, MSc1, Fatema Alzahrani, BSc1, Ruah Al-Yamany, MD1, Agaadir ALMoisheer, MSc1, Nour Ewida, BSc1, Shamsa Anazi, MSc1, Maha Alnemer, MD2, Mohamed Elsheikh, MD3, Khaled Alfaleh, MD3,4, Muneera Alshammari, MD4, Amal Alhashem, MD5, Abdullah A. Alangari, MD4, Mustafa A. Salih, MD4, Martin Kircher, MD6, Riza M. Daza, PhD6, Niema Ibrahim, BSc1, Salma M. Wakil, PhD1, Ahmed Alaqeel, MD7, Ikhlas Altowaijri, MD7, Jay Shendure, MD, PhD6, Amro Al-Habib, MD7, Eissa Faqieh, MD8 and Fowzan S. Alkuraya, MD1,9 Purpose: Dysmorphology syndromes are among the most common and C3ORF17). A significant minority of the phenotypes (6/31, 19%), referrals to clinical genetics specialists. Inability to match the dysmor- however, were caused by genes known to cause Mendelian pheno- phology pattern to a known syndrome can pose a major diagnostic chal- types, thus expanding the phenotypic spectrum of the diseases linked lenge. With an aim to accelerate the establishment of new syndromes to these genes. The conspicuous inheritance pattern and the highly and their genetic etiology, we describe our experience with multiplex specific phenotypes appear to have contributed to the high yield consanguineous families that appeared to represent novel autosomal (90%) of plausible molecular diagnoses in our study cohort. recessive dysmorphology syndromes at the time of evaluation. Conclusion: Reporting detailed clinical and genomic analysis of Methods: Combined autozygome/exome analysis of multiplex a large series of apparently novel dysmorphology syndromes will consanguineous families with apparently novel dysmorphology syn- likely lead to a trend to accelerate the establishment of novel syn- dromes. -

Supplementary Information For
Supplementary Information for An Abundance of Developmental Anomalies and Abnormalities in Pleistocene People Erik Trinkaus Department of Anthropology, Washington University, Saint Louis MO 63130 Corresponding author: Erik Trinkaus Email: [email protected] This PDF file includes: Supplementary text Figures S1 to S57 Table S1 References 1 to 421 for SI reference citations Introduction Although they have been considered to be an inconvenience for the morphological analysis of human paleontological remains, it has become appreciated that various pathological lesions and other abnormalities or rare variants in human fossil remains might provide insights into Pleistocene human biology and behavior (following similar trends in Holocene bioarcheology). In this context, even though there were earlier paleopathological assessments in monographic treatments of human remains (e.g., 1-3), it has become common to provide details on abnormalities in primary descriptions of human fossils (e.g., 4-12), as well as assessments of specific lesions on known and novel remains [see references in Wu et al. (13, 14) and below]. These works have been joined by doctoral dissertation assessments of patterns of Pleistocene human lesions (e.g., 15-18). The paleopathological attention has been primarily on the documentation and differential diagnosis of the abnormalities of individual fossil remains, leading to the growing paleopathological literature on Pleistocene specimens and their lesions. There have been some considerations of the overall patterns of the lesions, but those assessments have been concerned primarily with non-specific stress indicators and traumatic lesions (e.g., 13, 15, 19-21), with variable considerations of issues of survival 1 w ww.pnas.org/cgi/doi/10.1073/pnas.1814989115 and especially the inferred social support of the afflicted (e.g., 22-27). -

Lieshout Van Lieshout, M.J.S
EXPLORING ROBIN SEQUENCE Manouk van Lieshout Van Lieshout, M.J.S. ‘Exploring Robin Sequence’ Cover design: Iliana Boshoven-Gkini - www.agilecolor.com Thesis layout and printing by: Ridderprint BV - www.ridderprint.nl ISBN: 978-94-6299-693-9 Printing of this thesis has been financially supported by the Erasmus University Rotterdam. Copyright © M.J.S. van Lieshout, 2017, Rotterdam, the Netherlands All rights reserved. No parts of this thesis may be reproduced, stored in a retrieval system, or transmitted in any form or by any means without permission of the author or when appropriate, the corresponding journals Exploring Robin Sequence Verkenning van Robin Sequentie Proefschrift ter verkrijging van de graad van doctor aan de Erasmus Universiteit Rotterdam op gezag van de rector magnificus Prof.dr. H.A.P. Pols en volgens besluit van het College voor Promoties. De openbare verdediging zal plaatsvinden op woensdag 20 september 2017 om 09.30 uur door Manouk Ji Sook van Lieshout geboren te Seoul, Korea PROMOTIECOMMISSIE Promotoren: Prof.dr. E.B. Wolvius Prof.dr. I.M.J. Mathijssen Overige leden: Prof.dr. J.de Lange Prof.dr. M. De Hoog Prof.dr. R.J. Baatenburg de Jong Copromotoren: Dr. K.F.M. Joosten Dr. M.J. Koudstaal TABLE OF CONTENTS INTRODUCTION Chapter I: General introduction 9 Chapter II: Robin Sequence, A European survey on current 37 practice patterns Chapter III: Non-surgical and surgical interventions for airway 55 obstruction in children with Robin Sequence AIRWAY OBSTRUCTION Chapter IV: Unravelling Robin Sequence: Considerations 79 of diagnosis and treatment Chapter V: Management and outcomes of obstructive sleep 95 apnea in children with Robin Sequence, a cross-sectional study Chapter VI: Respiratory distress following palatal closure 111 in children with Robin Sequence QUALITY OF LIFE Chapter VII: Quality of life in children with Robin Sequence 129 GENERAL DISCUSSION AND SUMMARY Chapter VIII: General discussion 149 Chapter IX: Summary / Nederlandse samenvatting 169 APPENDICES About the author 181 List of publications 183 Ph.D. -

Identifying the Misshapen Head: Craniosynostosis and Related Disorders Mark S
CLINICAL REPORT Guidance for the Clinician in Rendering Pediatric Care Identifying the Misshapen Head: Craniosynostosis and Related Disorders Mark S. Dias, MD, FAAP, FAANS,a Thomas Samson, MD, FAAP,b Elias B. Rizk, MD, FAAP, FAANS,a Lance S. Governale, MD, FAAP, FAANS,c Joan T. Richtsmeier, PhD,d SECTION ON NEUROLOGIC SURGERY, SECTION ON PLASTIC AND RECONSTRUCTIVE SURGERY Pediatric care providers, pediatricians, pediatric subspecialty physicians, and abstract other health care providers should be able to recognize children with abnormal head shapes that occur as a result of both synostotic and aSection of Pediatric Neurosurgery, Department of Neurosurgery and deformational processes. The purpose of this clinical report is to review the bDivision of Plastic Surgery, Department of Surgery, College of characteristic head shape changes, as well as secondary craniofacial Medicine and dDepartment of Anthropology, College of the Liberal Arts characteristics, that occur in the setting of the various primary and Huck Institutes of the Life Sciences, Pennsylvania State University, State College, Pennsylvania; and cLillian S. Wells Department of craniosynostoses and deformations. As an introduction, the physiology and Neurosurgery, College of Medicine, University of Florida, Gainesville, genetics of skull growth as well as the pathophysiology underlying Florida craniosynostosis are reviewed. This is followed by a description of each type of Clinical reports from the American Academy of Pediatrics benefit from primary craniosynostosis (metopic, unicoronal, bicoronal, sagittal, lambdoid, expertise and resources of liaisons and internal (AAP) and external reviewers. However, clinical reports from the American Academy of and frontosphenoidal) and their resultant head shape changes, with an Pediatrics may not reflect the views of the liaisons or the emphasis on differentiating conditions that require surgical correction from organizations or government agencies that they represent. -

A Heads up on Craniosynostosis
Volume 1, Issue 1 A HEADS UP ON CRANIOSYNOSTOSIS Andrew Reisner, M.D., William R. Boydston, M.D., Ph.D., Barun Brahma, M.D., Joshua Chern, M.D., Ph.D., David Wrubel, M.D. Craniosynostosis, an early closure of the growth plates of the skull, results in a skull deformity and may result in neurologic compromise. Craniosynostosis is surprisingly common, occurring in one in 2,100 children. It may occur as an isolated abnormality, as a part of a syndrome or secondary to a systemic disorder. Typically, premature closure of a suture results in a characteristic cranial deformity easily recognized by a trained observer. it is usually the misshapen head that brings the child to receive medical attention and mandates treatment. This issue of Neuro Update will present the diagnostic features of the common types of craniosynostosis to facilitate recognition by the primary care provider. The distinguishing features of other conditions commonly confused with craniosynostosis, such as plagiocephaly, benign subdural hygromas of infancy and microcephaly, will be discussed. Treatment options for craniosynostosis will be reserved for a later issue of Neuro Update. Types of Craniosynostosis Sagittal synostosis The sagittal suture runs from the anterior to the posterior fontanella (Figure 1). Like the other sutures, it allows the cranial bones to overlap during birth, facilitating delivery. Subsequently, this suture is the separation between the parietal bones, allowing lateral growth of the midportion of the skull. Early closure of the sagittal suture restricts lateral growth and creates a head that is narrow from ear to ear. However, a single suture synostosis causes deformity of the entire calvarium. -

EUROCAT Syndrome Guide
JRC - Central Registry european surveillance of congenital anomalies EUROCAT Syndrome Guide Definition and Coding of Syndromes Version July 2017 Revised in 2016 by Ingeborg Barisic, approved by the Coding & Classification Committee in 2017: Ester Garne, Diana Wellesley, David Tucker, Jorieke Bergman and Ingeborg Barisic Revised 2008 by Ingeborg Barisic, Helen Dolk and Ester Garne and discussed and approved by the Coding & Classification Committee 2008: Elisa Calzolari, Diana Wellesley, David Tucker, Ingeborg Barisic, Ester Garne The list of syndromes contained in the previous EUROCAT “Guide to the Coding of Eponyms and Syndromes” (Josephine Weatherall, 1979) was revised by Ingeborg Barisic, Helen Dolk, Ester Garne, Claude Stoll and Diana Wellesley at a meeting in London in November 2003. Approved by the members EUROCAT Coding & Classification Committee 2004: Ingeborg Barisic, Elisa Calzolari, Ester Garne, Annukka Ritvanen, Claude Stoll, Diana Wellesley 1 TABLE OF CONTENTS Introduction and Definitions 6 Coding Notes and Explanation of Guide 10 List of conditions to be coded in the syndrome field 13 List of conditions which should not be coded as syndromes 14 Syndromes – monogenic or unknown etiology Aarskog syndrome 18 Acrocephalopolysyndactyly (all types) 19 Alagille syndrome 20 Alport syndrome 21 Angelman syndrome 22 Aniridia-Wilms tumor syndrome, WAGR 23 Apert syndrome 24 Bardet-Biedl syndrome 25 Beckwith-Wiedemann syndrome (EMG syndrome) 26 Blepharophimosis-ptosis syndrome 28 Branchiootorenal syndrome (Melnick-Fraser syndrome) 29 CHARGE -
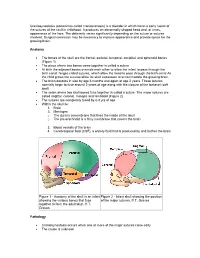
Craniosynostosis (Sometimes Called Craniostenosis) Is a Disorder in Which There Is Early Fusion of the Sutures of the Skull in Childhood
Craniosynostosis (sometimes called craniostenosis) is a disorder in which there is early fusion of the sutures of the skull in childhood. It produces an abnormally shaped head and, at times, appearance of the face. The deformity varies significantly depending on the suture or sutures involved. Surgical correction may be necessary to improve appearance and provide space for the growing brain. Anatomy • The bones of the skull are the frontal, parietal, temporal, occipital, and sphenoid bones (Figure 1) • The place where two bones come together is called a suture • At birth the adjacent bones override each other to allow the infant to pass through the birth canal. hinges called sutures, which allow the head to pass through the birth canal As the child grows the sutures allow for skull expansion to accommodate the growing brain. • The brain doubles in size by age 6 months and again at age 2 years. These sutures normally begin to fuse around 2 years of age along with the closure of the fontanel (soft spot) • The seam where two skull bones fuse together is called a suture. The major sutures are called sagittal, coronal, metopic and lambdoid (Figure 2) • The sutures are completely fused by 6-8 yrs of age • Within the skull lie: 1. Brain 2. Meninges o The dura is a membrane that lines the inside of the skull o The pia-arachnoid is a filmy membrane that covers the brain 3. Blood vessels of the brain 4. Cerebrospinal fluid (CSF), a watery fluid that is produced by and bathes the brain Figure 1 - Anatomy of the skull in an infant Figure 2 - Infant skull showing the position showing the various bones that fuse of the major sutures. -

Craniosynostosis
European Journal of Human Genetics (2011) 19, 369–376 & 2011 Macmillan Publishers Limited All rights reserved 1018-4813/11 www.nature.com/ejhg PRACTICAL GENETICS In association with Craniosynostosis Craniosynostosis, defined as the premature fusion of the cranial sutures, presents many challenges in classification and treatment. At least 20% of cases are caused by specific single gene mutations or chromosome abnormalities. This article maps out approaches to clinical assessment of a child presenting with an unusual head shape, and illustrates how genetic analysis can contribute to diagnosis and management. In brief Apart from the genetic implications, it is important to recog- nise cases with a genetic cause because they are more likely to Craniosynostosis is best managed in a multispecialty tertiary be associated with multiple suture synostosis and extracranial referral unit. complications. Single suture synostosis affects the sagittal suture most com- Genes most commonly mutated in craniosynostosis are monly, followed by the coronal, metopic and lambdoid sutures. FGFR2, FGFR3, TWIST1 and EFNB1. Both environmental factors (especially intrauterine fetal head As well as being associated with syndromes, some clinically constraint) and genes (single gene mutations, chromosome non-syndromic synostosis (usually affecting the coronal abnormalities and polygenic background) predispose to cra- suture) can be caused by single gene mutations, particularly niosynostosis. the Pro250Arg mutation in FGFR3. Most genetically determined craniosynostosis is characterised In severe cases, initial care should be directed towards main- by autosomal dominant inheritance, but around half of cases tenance of the airway, support of feeding, eye protection and are accounted for by new mutations. treatment of raised intracranial pressure. -

Craniosynostosis CRANIOSYNOSTOSIS
Craniosynostosis CRANIOSYNOSTOSIS Preoperative Postoperative Craniosynostosis can be defined as the sure has been found to be present in approxi- premature closing of one or more of the mately 13-23% of patients. normally present bony gaps between the different bones of the skull. These linear The surgical treatment of craniosynostosis areas where the bones have not yet healed has been transformed by the development and together are called cranial sutures. They applications of craniofacial techniques to intersect in certain areas of the skull form- reshape the skull and upper face in the infant. ing “soft spots” or fontanelles. The cranial Functionally, the goal in treatment is to release suture lines or non-ossified zones accom- the fused sutures to prevent any problems asso- modate the brain’s growth and expansion ciated with increased intracranial pressure as during the first years of life. The brain well as creating the potential for normal growth. increases in volume about 2-1/2 to 3 times Cosmetically, the goal is to obtain normal shape the first two years of life with these non- of the skull and face, thus minimizing psychoso- ossified areas of bone remaining open for a cial problems. variable length of time and then closing in a predictable manner. Premature synostosis or closure of the suture will stop the growth of the skull in the direction perpendicular to that suture; however, growth will contin- ue in the direction parallel to the suture. The result is that synostosis of a particular suture will alter the shape of the skull in a predictable manner with recognizable pat- terns. -

Perinatal/Neonatal Casebook &&&&&&&&&&&&&& Pfeiffer Syndrome, Type II
Perinatal/Neonatal Casebook &&&&&&&&&&&&&& Pfeiffer Syndrome, Type II Thomas E. Herman, MD described in patients with Crouzon, Apert, Carpenter, and Pfeiffer Marilyn J. Siegel, MD syndromes, and most commonly in Pfeiffer syndrome.6 The great- toe-to-second-toe ratio has been considered to be helpful in suggesting the diagnosis of Pfeiffer syndrome. This is made by measuring the maximum width of the first toe divided by the CASE PRESENTATION maximum width of the second toe. In Pfeiffer syndrome, this ratio A full-term child was born by caesarean section because of is approximately 2 (1.7 to 2.2). Values below 1.7 are not breech presentation to a 25-year-old gravida 1, para 0 mother. suggestive of the diagnosis.1,5 The pregnancy had been uncomplicated. The Apgar scores were Pfeiffer syndrome type II is a sporadic condition consisting of 8 at 1 minute and 9 at 5 minutes. The child was dysmorphic clover leaf skull, severe orbital proptosis, broad thumbs and great with a clover leaf skull, orbital proptosis, elbow and knee toes, and ankylosis of the elbows.1 Severe ocular proptosis and contractures, and an anteriorly displaced anus. Exposure hydrocephalus are the most common complications.1 Other keratopathy of the cornea was present and the child had a malformations include malpositioned anus, intestinal malrotation, temporary tarsorraphy (suturing of the eyelids) to allow this to pelvic kidney, bifid scrotum, widely spaced nipples, and choanal heal. A skeletal survey (Figure 1) and cranial sonogram stenosis.5 Type III is essentially the same as type II but without a (Figure 2) were also performed.