Roberto Caciuffo
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Badania Kazimierza Fajansa* W Dziedzinie Promieniotwórczości I Izotopii
A R TYKUŁY Józej Hurvoic (Marsylia) BADANIA KAZIMIERZA FAJANSA * W DZIEDZINIE PROMIENIOTWÓRCZOŚCI I IZOTOPII W 10 rocznicą śmierci Był to rok 1910. Sława 39-letniego wtedy Ernesta Rutherforda, już od dwóch lat laureata nagrody Nobla, ściągała do jego pracowni w Man chesterze badaczy z różnych krajów. Znajdowali się tu w tym okresie: Anglicy J. Chadwick, Charles Galton Darwin — wnuk słynnego twórcy teorii ewolucji, W. Makower, H. G. J. Moseley i J. M. Nuttal, Australij czyk D. C. H. Florance, Kanadyjczyk R. W. Boyle, Nowozelandczyk J. A. Gray, Amerykanin ze Stanów Zjednoczonych Alois F. Kowarik, Niemiec H. Geiger, Rosjanin G. N. Amltonow (Anitonoff) i Węgier G. von Hevesy. Do tego grona przybył Kazimierz Fajans. Urodzony 27 maja 1887 ro ku w Warszawie, po ukończeniu tamże gimnazjum studiował chemię na uniwersytecie w Lipsku, następnie w Heidelbergu uzyskał doktorat za pracę z zakresu chemii fizycznej organicznej wykonaną pod opieką pro fesora Georga Brediga, a w Zurychu odbył uzupełniający staż podoklor ski w dziedzinie chemii organicznej. Tam jednak, stwierdziwszy, że ze względu na empiryczny charakter ówczesnej chemii organicznej nie znajduje upodobania w tej gałęzi wiedzy, postanawia zająć się badaniem promieniotwórczości. Wraz ze świeżo poślubioną Salomeą Kapłan, war szawianką studiującą na uniwersytecie w Heidelbergu, wybiera się na rok akademicki 1910—1911 do Manchesteru. Najbliższym współpracownikiem Rutherforda był wtedy Hans Geiger, którego imię nosi licznik do pomiarów promieniotwórczości, wynalezio ny w 1912 r., a później przy udziale W. Müllera ulepszony. Dla Fajansa Geiger stał się głównym przewodnikiem i doradcą. Złożyły się na to * ur. 27 maja 1887 r., Warszawa — zm. 18 maja 1975 r., Ann Arbor. -

Sterns Lebensdaten Und Chronologie Seines Wirkens
Sterns Lebensdaten und Chronologie seines Wirkens Diese Chronologie von Otto Sterns Wirken basiert auf folgenden Quellen: 1. Otto Sterns selbst verfassten Lebensläufen, 2. Sterns Briefen und Sterns Publikationen, 3. Sterns Reisepässen 4. Sterns Züricher Interview 1961 5. Dokumenten der Hochschularchive (17.2.1888 bis 17.8.1969) 1888 Geb. 17.2.1888 als Otto Stern in Sohrau/Oberschlesien In allen Lebensläufen und Dokumenten findet man immer nur den VornamenOt- to. Im polizeilichen Führungszeugnis ausgestellt am 12.7.1912 vom königlichen Polizeipräsidium Abt. IV in Breslau wird bei Stern ebenfalls nur der Vorname Otto erwähnt. Nur im Emeritierungsdokument des Carnegie Institutes of Tech- nology wird ein zweiter Vorname Otto M. Stern erwähnt. Vater: Mühlenbesitzer Oskar Stern (*1850–1919) und Mutter Eugenie Stern geb. Rosenthal (*1863–1907) Nach Angabe von Diana Templeton-Killan, der Enkeltochter von Berta Kamm und somit Großnichte von Otto Stern (E-Mail vom 3.12.2015 an Horst Schmidt- Böcking) war Ottos Großvater Abraham Stern. Abraham hatte 5 Kinder mit seiner ersten Frau Nanni Freund. Nanni starb kurz nach der Geburt des fünften Kindes. Bald danach heiratete Abraham Berta Ben- der, mit der er 6 weitere Kinder hatte. Ottos Vater Oskar war das dritte Kind von Berta. Abraham und Nannis erstes Kind war Heinrich Stern (1833–1908). Heinrich hatte 4 Kinder. Das erste Kind war Richard Stern (1865–1911), der Toni Asch © Springer-Verlag GmbH Deutschland 2018 325 H. Schmidt-Böcking, A. Templeton, W. Trageser (Hrsg.), Otto Sterns gesammelte Briefe – Band 1, https://doi.org/10.1007/978-3-662-55735-8 326 Sterns Lebensdaten und Chronologie seines Wirkens heiratete. -

Inorganic Chemistry for Dummies® Published by John Wiley & Sons, Inc
Inorganic Chemistry Inorganic Chemistry by Michael L. Matson and Alvin W. Orbaek Inorganic Chemistry For Dummies® Published by John Wiley & Sons, Inc. 111 River St. Hoboken, NJ 07030-5774 www.wiley.com Copyright © 2013 by John Wiley & Sons, Inc., Hoboken, New Jersey Published by John Wiley & Sons, Inc., Hoboken, New Jersey Published simultaneously in Canada No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, scanning or otherwise, except as permitted under Sections 107 or 108 of the 1976 United States Copyright Act, without either the prior written permis- sion of the Publisher, or authorization through payment of the appropriate per-copy fee to the Copyright Clearance Center, 222 Rosewood Drive, Danvers, MA 01923, (978) 750-8400, fax (978) 646-8600. Requests to the Publisher for permission should be addressed to the Permissions Department, John Wiley & Sons, Inc., 111 River Street, Hoboken, NJ 07030, (201) 748-6011, fax (201) 748-6008, or online at http://www.wiley. com/go/permissions. Trademarks: Wiley, the Wiley logo, For Dummies, the Dummies Man logo, A Reference for the Rest of Us!, The Dummies Way, Dummies Daily, The Fun and Easy Way, Dummies.com, Making Everything Easier, and related trade dress are trademarks or registered trademarks of John Wiley & Sons, Inc. and/or its affiliates in the United States and other countries, and may not be used without written permission. All other trade- marks are the property of their respective owners. John Wiley & Sons, Inc., is not associated with any product or vendor mentioned in this book. -

February 2020 Newsletter Manhattan Project NHP Oak Ridge
National Park Service February 2020 Department of the Interior Manhattan Project National Historical Park Oak Ridge, Tennessee Manhattan Project History in February Visit Inside the Oak Ridge Turnpike Gatehouse on Sat- Philip Abelson began working on uranium enrichment urday, February 8 at 3:30 pm (ET). Rangers will host the using liquid thermal diffusion in February of 1941. How- popular “Secrecy, Security, and Spies” program at the Turn- ever construction of his thermal diffusion plant in Phila- pike Gatehouse. The program will begin at 3:30 pm (ET) giv- delphia did not begin until January 1944. This same pro- ing visitors some insight to what life was like in Oak Ridge cess would be used later at the S-50 plant in Oak Ridge, during the Manhattan Project with the need for secrecy, all TN. of the security that surrounded the city, and the threat of Glenn Seaborg and his research group discovered Pluto- spies. The Turnpike Gatehouse is located at 2900 Oak Ridge nium while working at the University of California, Berke- Turnpike, Oak Ridge, TN 37830. ley on February 24, 1941. Construction of the -Y 12 Ura- Join a Park Ranger for a nium enrichment facilities and “Manhattan Project in Pop the X-10 Graphite Reactor be- Culture” on Saturday, Febru- gan in February 1943. Stone ary 22 at 3 pm (ET) at the Ameri- and Webster Engineering was can Museum of Science & Energy, in charge of Y-12 construction 115 East Main Street, Oak Ridge. while DuPont handled X-10. We will lead a discussion of the One year after construction Y- impact the Project had on popu- 12 sent 200 grams of uranium- lar culture in movies, music and 235 to Los Alamos, NM in Feb- television. -

Science Loses Some Friends Francis Crick, Thomas Gold, and Philip Abelson
Monthly Planet October 2004 Science Loses Some Friends Francis Crick, Thomas Gold, and Philip Abelson By Iain Murray he scientifi c world lost three impor- Throughout his career, Abelson used which suggests that the universe and Ttant fi gures in recent weeks, as scientifi c principles to determine genu- the laws of physics have always existed Francis Crick, Thomas Gold, and Philip ine new developments from hype and in the same, steady state. This has since Abelson have all passed away. In their publicity stunts (he was famously dis- been supplanted as the dominant cos- careers, each demonstrated the best that missive of the scientifi c value of the race mological paradigm by the Big Bang science has to offer humanity. Their loss to the moon). In that, he should prove a theory. illustrates how much worse off the state role model for true scientists. After working at the Royal Greenwich of science is today than during their Observatory, Gold moved to the United glory years. Thomas Gold States to become Professor of Astron- Astronomer Thomas Gold had an omy at Harvard. From there he moved Philip Abelson equally distinguished career, in fi elds as to Cornell, where he demonstrated that Philip Abelson was a scientist of truly diverse as engineering, physiology, and the newly discovered “pulsar” phenom- broad talents. One of America’s fi rst cosmology; and he was never afraid of enon must contain a rotating neutron nuclear physicists, he discovered the being called a maverick. A fellow of both star (a star more massive than the sun element Neptunium and designed the the Royal Society and the National Acad- but just 10 km in diameter). -

Sylwetka Naukowa Kazimierza Fajansa
POLSKA AKADEMIA UMIEJĘTNOŚCI TOM II PRACE KOMISJI HISTORII NAUKI 2000 Józef HURWIC SYLWETKA NAUKOWA KAZIMIERZA FAJANSA Kazimierz Fajans był jednym z najwybitniejszych fizykochemików pierw szej połowy naszego stulecia. W 1929 r. został wybrany na członka czynnego Wydziału Matematyczno-Przyrodniczego Polskiej Akademii Umiejętności. Mimo dużej różnicy wieku miałem zaszczyt i szczęście utrzymywać z nim i jego rodziną stosunki przyjaźni. W 1991 r. Instytut Historii Nauki, Oświaty i Techniki PAN wydał nakładem Ossolineum opracowaną przeze mnie dość obszerną biografię Fajansa1 do której odsyłam bliżej zainteresowanych jego osobą i dziełem. W niniejszym referacie postaram się zwięźle przedstawić najważniejsze jego dokonania naukowe. Kazimierz Fajans urodził się 27 maja 1887 r. w Warszawie w spolonizo wanej rodzinie żydowskiej bardzo dla Polski zasłużonej. Po ukończeniu w 1904 r. szkoły średniej w rodzinnym mieście wyjeżdża na studia chemiczne do Lipska, które następnie kontynuuje w Heidelbergu. Tu pod opieką Georga Brediga wykonuje pracę doktorską na temat katalizy stereospecyficznej2. Pracę obronił w 1909 r. Do jej tematu więcej nie wrócił. Bardziej zainteresowała go promie niotwórczość. W roku akademickim 1910-1911 odbył staż w sławnej pracowni Ernesta Rutherforda w Manchesterze. Przebywali tam wówczas m.in.: James Chadwick, Hans Geiger, Georg von Hevesy, H. J. G. Moseley, J. M. Nuttal. Fajans znalazł się w centrum badań, które w 1911 r. doprowadziły Rutherforda do odkrycia jądra atomowego na podstawie analizy rozpraszania cząstek a przez cienką folię metalową3. Fajans był obecny na historycznym posiedzeniu Man- chesterskiego Towarzystwa Filozoficznego, gdy Rutherford referował to epoko we odkrycie. Na sali znajdowali się przeważnie młodzi rówieśnicy Fajansa. Po 1 J. Hurwic, Kazimierz Fajans (1887-1975). Sylwetka Uczonego, Ossolineum, Wro- cław-Warszawa-Kraków-Gdańsk-Łódź 1991. -

Ija, LLP Alan D
20-12212-mew Doc 1196 Filed 05/07/21 Entered 05/07/21 21:22:15 Main Document Pg 1 of 74 UNITED STATES BANKRUPTCY COURT SOUTHERN DISTRICT OF NEW YORK -----------------------------------------------------------------------x : In re: : Chapter 11 : GARRETT MOTION INC., et al.,1 : Case No. 20-12212 (MEW) : Debtors. : (Jointly Administered) : -----------------------------------------------------------------------x CERTIFICATE OF SERVICE I, Rossmery Martinez, depose and say that I am employed by Kurtzman Carson Consultants LLC (KCC), the claims and noticing agent for the Debtors in the above-captioned case. On May 4, 2021, at my direction and under my supervision, employees of KCC caused to be served the following document via Electronic Mail upon the service list attached hereto as Exhibit A; and via First Class Mail upon the service list attached hereto as Exhibit B: Notice of June Omnibus Hearing Date [Docket No. 1192] Furthermore, on May 4, 2021, at my direction and under my supervision, employees of KCC caused to be served the following document via Electronic Mail upon the service list attached hereto as Exhibit A; and via First Class Mail upon the service lists attached hereto as Exhibit B and Exhibit C: (Continued on Next Page) 1 The last four digits of Garrett Motion Inc.’s tax identification number are 3189. Due to the large number of debtor entities in these Chapter 11 Cases, which are being jointly administered, a complete list of the Debtors and the last four digits of their federal tax identification numbers is not provided herein. A complete list of such information may be obtained on the website of the Debtors’ claims and noticing agent at http://www.kccllc.net/garrettmotion. -
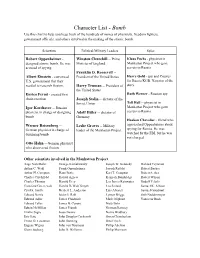
Character List
Character List - Bomb Use this chart to help you keep track of the hundreds of names of physicists, freedom fighters, government officials, and others involved in the making of the atomic bomb. Scientists Political/Military Leaders Spies Robert Oppenheimer - Winston Churchill -- Prime Klaus Fuchs - physicist in designed atomic bomb. He was Minister of England Manhattan Project who gave accused of spying. secrets to Russia Franklin D. Roosevelt -- Albert Einstein - convinced President of the United States Harry Gold - spy and Courier U.S. government that they for Russia KGB. Narrator of the needed to research fission. Harry Truman -- President of story the United States Enrico Fermi - created first Ruth Werner - Russian spy chain reaction Joseph Stalin -- dictator of the Tell Hall -- physicist in Soviet Union Igor Korchatov -- Russian Manhattan Project who gave physicist in charge of designing Adolf Hitler -- dictator of secrets to Russia bomb Germany Haakon Chevalier - friend who Werner Reisenberg -- Leslie Groves -- Military approached Oppenheimer about German physicist in charge of leader of the Manhattan Project spying for Russia. He was designing bomb watched by the FBI, but he was not charged. Otto Hahn -- German physicist who discovered fission Other scientists involved in the Manhattan Project: Aage Niels Bohr George Kistiakowsky Joseph W. Kennedy Richard Feynman Arthur C. Wahl Frank Oppenheimer Joseph Rotblat Robert Bacher Arthur H. Compton Hans Bethe Karl T. Compton Robert Serber Charles Critchfield Harold Agnew Kenneth Bainbridge Robert Wilson Charles Thomas Harold Urey Leo James Rainwater Rudolf Pelerls Crawford Greenewalt Harold DeWolf Smyth Leo Szilard Samuel K. Allison Cyril S. Smith Herbert L. Anderson Luis Alvarez Samuel Goudsmit Edward Norris Isidor I. -

Feb 05 Nucleus Corrected
DED UN 18 O 98 F yyyy N yyyy Y O T R E I T H C E N O yyyy A E S S S L T A E A C R C I yyyyN S M S E E H C C T N IO A February 2005 Vol. LXXXIII, No. 6 yyyyC N • AMERI Monthly Meeting Prof. Shana Kelley on Nanotechnological and Biomolecules Arno Heyn Dedicated NESACS Member Organohalogens Gordon Gribble Writes Philip Abelson A National Figure in Science Call for Papers 20052005 ❖ Deadline – April 15, 2005 ❖ EASTERN November 14-17, 2005 ANALYTICAL Garden State Exhibit Center Somerset, NJ SYMPOSIUM he Eastern Analytical Symposium is the second largest meeting in the United States dedicated to the needs Innovation through Education of analytical chemists and those in the allied sciences. Please help us to make the 2005 EAS the best ever–be a part of the program by contributing your own papers for inclusion in the oral or poster sessions. To submit a contributed presentation for the 2005 EAS Technical Program, you should go to our web site, www.eas.org, after March 1, and follow the instructions for preliminary abstract submission. Invited speakers should not submit preliminary abstracts to EAS, although your session organizer may request one for his/her use. All preliminary abstracts must be submitted elec- tronically via the EAS web site at www.eas.org. The abstract submis- sion deadline is April 15, 2005. No faxed, e-mailed, or mailed prelimi- nary abstracts will be accepted. Please carefully review the following information: 1. -

Listening to Black Holes
Issue 03 Feb 2017 REDISCOVERING SCHOOL SCIENCE Page 4 LISTENING TO BLACK HOLES A publication from Azim Premji University i wonder No. 134, Doddakannelli Next to Wipro Corporate Office Sarjapur Road, Bangalore - 560 035. India Tel: +91 80 6614 9000/01/02 Fax: +91 806614 4903 www.azimpremjifoundation.org Also visit Azim Premji University website at www.azimpremjiuniversity.edu.in A soft copy of this issue can be downloaded from www.http://azimpremjiuniversity.edu.in/SitePages/resources-iwonder.aspx Editors Ramgopal (RamG) Vallath Chitra Ravi Editorial Committee REDISCOVERING SCHOOL SCIENCE Anand Narayanan Chandrika Muralidhar Geetha Iyer Hridaykant Dewan Editor’s Desk Jayalekshmy Ayyer Julfikar Ali Propelled by science, humanity is at the cusp of becoming a spacefaring Radha Gopalan civilisation, with ‘eyes’ firmly set on distant stars and feet taking the first Rajaram Nityananda baby steps towards other planets in the Solar system. At this juncture, it Reeteka Sud is important to remember that science education can no longer be limited Richard Fernandes to telling students a plethora of facts, as if they are a given. Rather, the role of science education, as emphasized in the article Why Science Saurav Shome Matters, is to enable children understand and appreciate the scientific Sushil Joshi process that has led to the most plausible hypotheses being proven with Yasmin Jayathirtha hard data and then being presented as theories. It is also about Advisors encouraging children to make use of this process, not only in scientific Falguni Sarangi quests, but also in their day-to-day life. Effective science teachers can convey the beauty of this process and share the wonder it brings with Manoj P their students. -

National Park Service Department of the Interior
National Park Service May 2017 Department of the Interior Manhattan Project National Historical Park Oak Ridge, Tennessee May in MAPR History April Showers Brings May Flowers and Also Cabbage, British physicist James Chadwick would later be awarded the Tomatoes and Green Beans. We are hard at work in the Nobel Prize in Physics for his discovery of the neutron in May Victory Garden next to the Flat Top House with students 1932. from Willow Brook Elementary every Wednesday. Students Gorge Kistiakowsky and Vannevar Bush have been planting and caring for the garden discussed the possibility of producing U- and learning about war time rationing. 235 by using gaseous diffusion in May 1940. On May 27, 1940, the discovery of radioac- Learn about the Upcoming Total Solar tive element 93, neptunium, is announced Eclipse on Thursday, May 18. Join us for a in a report submitted by Edwin McMillan lecture featuring Chap Percival who will and Philip Abelson. The same day Leo Szil- speak on the upcoming August 21 solar ard received a manuscript from Louis eclipse. This free lecture will be held at the Turner arguing that the isotope of element American Museum of Science and Energy. A 94, still undiscovered plutonium, should reception will start at 5:30 p.m. (ET) and the be highly fissionable like uranium-235. lecture starts at 6 p.m. Glenn Seaborg was able to prove on May 3, 1941, that plutonium-239 was more fissionable than uranium-235 through his This Month’s AMSE Curatorial Collection research at the University of California, Highlight is Jack Beers’ Library Card from Berkeley. -

Edwin M. Mcmillan, a Biographical Sketch
LBL 35965 HQDFAN668 EDWIN M. MCMILLAN, A BIOGRAPHICAL SKETCH EDWARD J. LOFGREN, Associate Director Emeritus LAWRENCE BERKELEY LABORATORY BERKELEY, CALIFORNIA 94720 Presented at the International Symposium: The 50th Anniversary of the Phase Stability Principle Moscow/Dubna, Russia 12-15 July 1994 ABSTRACT. Edwin M. McMillan was one of the great scientists of the middle years of this century. He made notable contributions to nuclear, and particle physics, the chemis try of transuranic elements, and accelerator physics. * This work supported by the Director, Office of Energy Research, Office of Fusion Energy, U.S. Department of Energy under contract No. DE-AC03-76SF00098. ^-TWBUTION OF THIS DOCUMENT (S UNLIMITED Edwin McMillan was born on Septem tion Laboratory. He joined the Laboratory in ber 18,1907 at Redondo Beach, California. Soon 1934 and began a lifelong close association with after, the family moved to Pasadena, California Lawrence. where the father practised medicine for many He quickly established himself as a me years. The move to Pasadena was a very fortu ticulous and versatile experimenter in nuclear nate one because Caltech was nearby and even physics with an excellent grasp of theory. Dur in his early school years Ed could take advan ing this period he discovered *-0 with Stanley tage of the excellent public programs and lec Livingston and 10Be with Samuel Ruben. Per tures put on there by them. This contact with the haps his best experiment at that time was the world of science as Ed grew from boyhood to a demonstration of electron pair production by the young man was very important, for it nurtured absorption of gamma rays from fluorine bom the wide and lasting curiosity and interest that barded by protons from the cyclotron.