The Optic Nerve Head; a Review of the Normal Variants, Disc Anomalies and Optic Nerve Disorders That May Be Encountered During Retinal Screening Clinics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Optic Disc Edema, Globe Flattening, Choroidal Folds, and Hyperopic Shifts Observed in Astronauts After Long-Duration Space Flight
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln NASA Publications National Aeronautics and Space Administration 10-2011 Optic Disc Edema, Globe Flattening, Choroidal Folds, and Hyperopic Shifts Observed in Astronauts after Long-duration Space Flight Thomas H. Mader Alaska Native Medical Center, [email protected] C. Robert Gibson Coastal Eye Associates Anastas F. Pass University of Houston Larry A. Kramer University of Texas Health Science Center Andrew G. Lee The Methodist Hospital See next page for additional authors Follow this and additional works at: https://digitalcommons.unl.edu/nasapub Part of the Physical Sciences and Mathematics Commons Mader, Thomas H.; Gibson, C. Robert; Pass, Anastas F.; Kramer, Larry A.; Lee, Andrew G.; Fogarty, Jennifer; Tarver, William J.; Dervay, Joseph P.; Hamilton, Douglas R.; Sargsyan, Ashot; Phillips, John L.; Tran, Duc; Lipsky, William; Choi, Jung; Stern, Claudia; Kuyumjian, Raffi; andolk, P James D., "Optic Disc Edema, Globe Flattening, Choroidal Folds, and Hyperopic Shifts Observed in Astronauts after Long-duration Space Flight" (2011). NASA Publications. 69. https://digitalcommons.unl.edu/nasapub/69 This Article is brought to you for free and open access by the National Aeronautics and Space Administration at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in NASA Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Authors Thomas H. Mader, C. Robert Gibson, Anastas F. Pass, Larry A. -

Bass – Glaucomatous-Type Field Loss Not Due to Glaucoma
Glaucoma on the Brain! Glaucomatous-Type Yes, we see lots of glaucoma Field Loss Not Due to Not every field that looks like glaucoma is due to glaucoma! Glaucoma If you misdiagnose glaucoma, you could miss other sight-threatening and life-threatening Sherry J. Bass, OD, FAAO disorders SUNY College of Optometry New York, NY Types of Glaucomatous Visual Field Defects Paracentral Defects Nasal Step Defects Arcuate and Bjerrum Defects Altitudinal Defects Peripheral Field Constriction to Tunnel Fields 1 Visual Field Defects in Very Early Glaucoma Paracentral loss Early superior/inferior temporal RNFL and rim loss: short axons Arcuate defects above or below the papillomacular bundle Arcuate field loss in the nasal field close to fixation Superotemporal notch Visual Field Defects in Early Glaucoma Nasal step More widespread RNFL loss and rim loss in the inferior or superior temporal rim tissue : longer axons Loss stops abruptly at the horizontal raphae “Step” pattern 2 Visual Field Defects in Moderate Glaucoma Arcuate scotoma- Bjerrum scotoma Focal notches in the inferior and/or superior rim tissue that reach the edge of the disc Denser field defects Follow an arcuate pattern connected to the blind spot 3 Visual Field Defects in Advanced Glaucoma End-Stage Glaucoma Dense Altitudinal Loss Progressive loss of superior or inferior rim tissue Non-Glaucomatous Etiology of End-Stage Glaucoma Paracentral Field Loss Peripheral constriction Hereditary macular Loss of temporal rim tissue diseases Temporal “islands” Stargardt’s macular due -

Bilateral Acquired Progressive Retinal Nerve Fiber Layer Myelination
Bilateral Acquired Progressive Retinal Nerve Fiber Layer Myelination Abstract We present the multimodal imaging findings of an unusual case of bilateral acquired progressive myelination of the optic disc over a 10-year follow up period, in a hyperopic adolescent patient in the absence of an underlying ocular or systemic abnormality. Myelination of the left optic disc was noted at age 7 and of the right optic disc at age 13 but no other ocular or systemic abnormalities were identified. Cross sectional OCT and en face OCT angiography confirmed the presence of myelination of the retinal nerve fiber layer and excluded other etiologic possibilities including an astrocytic hamartoma. Keywords: Optic Nerve; Optic Fiber Myelination, Acquired, Hyperopia INTRODUCTION Myelination of the retinal nerve fiber layer occurs in 1% of the population and is most frequently congenital and non-progressive. (1) Congenital cases are typically isolated but may be rarely associated with the syndrome of ipsilateral high myopia, strabismus and amblyopia (2,3), although cases have been reported in hyperopic eyes. Reports of acquired and progressive myelination associated with congenital optic nerve disorders such as Arnold-Chiari malformation, hydrocephalus, optic disc drusen, and iatrogenic causes such as optic nerve sheath fenestration, are relatively rare.(4–8) PLEASE ADJUST THE REFERENCE CITATIONS While affected patients are typically asymptomatic, retinal nerve fiber layer myelination can be rarely associated with visual loss. Even more unusual are reports of acquired and progressive myelination of the nerve fiber layer. We present the multimodal imaging findings of such a case in a healthy young patient with a 10-year follow up. -

98796-Anatomy of the Orbit
Anatomy of the orbit Prof. Pia C Sundgren MD, PhD Department of Diagnostic Radiology, Clinical Sciences, Lund University, Sweden Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lay-out • brief overview of the basic anatomy of the orbit and its structures • the orbit is a complicated structure due to its embryological composition • high number of entities, and diseases due to its composition of ectoderm, surface ectoderm and mesoderm Recommend you to read for more details Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 3 x 3 Imaging technique 3 layers: - neuroectoderm (retina, iris, optic nerve) - surface ectoderm (lens) • CT and / or MR - mesoderm (vascular structures, sclera, choroid) •IOM plane 3 spaces: - pre-septal •thin slices extraconal - post-septal • axial and coronal projections intraconal • CT: soft tissue and bone windows 3 motor nerves: - occulomotor (III) • MR: T1 pre and post, T2, STIR, fat suppression, DWI (?) - trochlear (IV) - abducens (VI) Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Superior orbital fissure • cranial nerves (CN) III, IV, and VI • lacrimal nerve • frontal nerve • nasociliary nerve • orbital branch of middle meningeal artery • recurrent branch of lacrimal artery • superior orbital vein • superior ophthalmic vein Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lund University / Faculty of Medicine / Inst. -
Eye60. Instrumental Eye Examination.Pdf
INSTRUMENTAL EYE EXAMINATION Eye60 (1) Instrumental Eye Examination Last updated: May 9, 2019 “BEDSIDE” EXAMINATIONS ..................................................................................................................... 1 OPHTHALMOSCOPY (FUNDUSCOPY) ........................................................................................................ 1 DIRECT OPHTHALMOSCOPY .................................................................................................................... 1 INDIRECT OPHTHALMOSCOPY ................................................................................................................. 2 OPHTHALMOSCOPIC FINDINGS ............................................................................................................... 2 Hypertensive retinopathy ................................................................................................................. 6 Diabetic retinopathy ......................................................................................................................... 7 PEDIATRIC ASPECTS ............................................................................................................................... 9 APPLANATION TONOMETRY .................................................................................................................... 9 SLIT LAMP EXAMINATION (BIOMICROSCOPY) ........................................................................................ 9 ULTRASONOGRAPHY .............................................................................................................................. -

Anatomy and Physiology of the Afferent Visual System
Handbook of Clinical Neurology, Vol. 102 (3rd series) Neuro-ophthalmology C. Kennard and R.J. Leigh, Editors # 2011 Elsevier B.V. All rights reserved Chapter 1 Anatomy and physiology of the afferent visual system SASHANK PRASAD 1* AND STEVEN L. GALETTA 2 1Division of Neuro-ophthalmology, Department of Neurology, Brigham and Womens Hospital, Harvard Medical School, Boston, MA, USA 2Neuro-ophthalmology Division, Department of Neurology, Hospital of the University of Pennsylvania, Philadelphia, PA, USA INTRODUCTION light without distortion (Maurice, 1970). The tear–air interface and cornea contribute more to the focusing Visual processing poses an enormous computational of light than the lens does; unlike the lens, however, the challenge for the brain, which has evolved highly focusing power of the cornea is fixed. The ciliary mus- organized and efficient neural systems to meet these cles dynamically adjust the shape of the lens in order demands. In primates, approximately 55% of the cortex to focus light optimally from varying distances upon is specialized for visual processing (compared to 3% for the retina (accommodation). The total amount of light auditory processing and 11% for somatosensory pro- reaching the retina is controlled by regulation of the cessing) (Felleman and Van Essen, 1991). Over the past pupil aperture. Ultimately, the visual image becomes several decades there has been an explosion in scientific projected upside-down and backwards on to the retina understanding of these complex pathways and net- (Fishman, 1973). works. Detailed knowledge of the anatomy of the visual The majority of the blood supply to structures of the system, in combination with skilled examination, allows eye arrives via the ophthalmic artery, which is the first precise localization of neuropathological processes. -

Using the Ophthalmoscope: Viewing the Optic Disc and Retina
Using the Ophthalmoscope: Viewing the Optic Disc and Retina Judith Warner, MD University of Utah THE OPHTHALMOSCOPE DIRECT OPHTHALMOSCOPY • Jan Purkinje 1823 • Hermann von Helmholtz 1851 • Hand held ophthalmoscope • Direct up-right image Dials of the Ophthalmoscope RED-FREE FILTER (GREEN LIGHT) 450 nm monochromatic light nerve fiber layer optic nerve drusen OTHER DIALS • Used for measuring lesion size • Looking for the center of fixation OTHER DIALS: SLIT BEAM The wheel has lenses of power Panoptic-ophthalmoscope Direct type Wider field of view Distance from pt greater Similar apertures Not as easy to carry Slightly dimmer light source Not as magnified view of Disc Clean the rubber cup between patients Photographs: http://panoptic.welchallyn.com/faq.html WHEN EVER POSSIBLE: DILATE THE PATIENT Steps to Direct Ophthalmoscopy • Dimly lit room • Dilating drops • Patient fixates distant target • Align yourself • Red reflex • Dial in HOW TO USE THE DIRECT Ophthalmoscope.avi ophthalmoscope.wmv THE RED REFLEX The layers you will go through to see the optic disc THE OPTIC NERVE WHAT YOU SHOULD OBSERVE IN EVERYONE RIGHT EYE AND LEFT EYE THE NORMAL DISC • The disc is 1.62 mm or 1 million fibers • Central retinal artery and vein • Lamina Cribrosa • The optic cup The Normal Disc Appearance The lamina cribrosa is an important disc structure --Means Sieve --Anatomically present in all discs --Visible in about 1/3 --Shallow in myopia Look at the Cup-to-disc ratio: WHAT IS THE CUP-TO-DISC RATIO? .7 NO CUP 0.1 CUP 0.3 CUP 0.7 CUP 0.9 CUP What is the cup -

Central Serous Papillopathy by Optic Nerve Head Drusen
Clinical Ophthalmology Dovepress open access to scientific and medical research Open Access Full Text Article CASE REPORT Central serous papillopathy by optic nerve head drusen Ana Marina Suelves1 Abstract: We report a 38-year-old man with a complaint of blurred vision in his right eye for the Ester Francés-Muñoz1 previous 5 days. He had bilateral optic disc drusen. Fluorescein angiography revealed multiple Roberto Gallego-Pinazo1 hyperfluorescent foci within temporal optic discs and temporal inferior arcade in late phase. Diamar Pardo-Lopez1 Optical coherence tomography showed bilateral peripapillary serous detachment as well as right Jose Luis Mullor2 macular detachment. This is the first reported case of a concurrent peripapillary and macular Jose Fernando Arevalo3 detachment in a patient with central serous papillopathy by optic disc drusen. Central serous papillopathy is an atypical form of central serous chorioretinopathy that should be considered Manuel Díaz-Llopis1,4,5 as a potential cause of acute loss of vision in patients with optic nerve head drusen. 1 Department of Ophthalmology, La Fe Keywords: central serous papillopathy, peripapillary central serous chorioretinopathy, optic University Hospital, Valencia, Spain; For personal use only. 2Instituto de Investigación Sanitaria, nerve head drusen, peripapillary subretinal fluid Fundación para la investigación, La Fe Hospital, Valencia, Spain; 3Retina and vitreous service, Clínica Introduction Oftalmológica Centro Caracas, Optic nerve head drusen (ONHD) are hyaline material calcificated -

The Horizontal Raphe of the Human Retina and Its Watershed Zones
vision Review The Horizontal Raphe of the Human Retina and its Watershed Zones Christian Albrecht May * and Paul Rutkowski Department of Anatomy, Medical Faculty Carl Gustav Carus, TU Dresden, 74, 01307 Dresden, Germany; [email protected] * Correspondence: [email protected] Received: 24 September 2019; Accepted: 6 November 2019; Published: 8 November 2019 Abstract: The horizontal raphe (HR) as a demarcation line dividing the retina and choroid into separate vascular hemispheres is well established, but its development has never been discussed in the context of new findings of the last decades. Although factors for axon guidance are established (e.g., slit-robo pathway, ephrin-protein-receptor pathway) they do not explain HR formation. Early morphological organization, too, fails to establish a HR. The development of the HR is most likely induced by the long posterior ciliary arteries which form a horizontal line prior to retinal organization. The maintenance might then be supported by several biochemical factors. The circulation separate superior and inferior vascular hemispheres communicates across the HR only through their anastomosing capillary beds resulting in watershed zones on either side of the HR. Visual field changes along the HR could clearly be demonstrated in vascular occlusive diseases affecting the optic nerve head, the retina or the choroid. The watershed zone of the HR is ideally protective for central visual acuity in vascular occlusive diseases but can lead to distinct pathological features. Keywords: anatomy; choroid; development; human; retina; vasculature 1. Introduction The horizontal raphe (HR) was first described in the early 1800s as a horizontal demarcation line that extends from the macula to the temporal Ora dividing the temporal retinal nerve fiber layer into a superior and inferior half [1]. -

Molecular Regulation of Visual System Development: More Than Meets the Eye
Downloaded from genesdev.cshlp.org on September 30, 2021 - Published by Cold Spring Harbor Laboratory Press REVIEW Molecular regulation of visual system development: more than meets the eye Takayuki Harada,1,2 Chikako Harada,1,2 and Luis F. Parada1,3 1Department of Developmental Biology and Kent Waldrep Foundation Center for Basic Neuroscience Research on Nerve Growth and Regeneration, University of Texas Southwestern Medical Center, Dallas, Texas 75235, USA; 2Department of Molecular Neurobiology, Tokyo Metropolitan Institute for Neuroscience, Fuchu, Tokyo 183-8526, Japan Vertebrate eye development has been an excellent model toderm, intercalating mesoderm, surface ectoderm, and system to investigate basic concepts of developmental neural crest (Fig. 1). The neuroectoderm differentiates biology ranging from mechanisms of tissue induction to into the retina, iris, and optic nerve; the surface ecto- the complex patterning and bidimensional orientation of derm gives rise to lens and corneal epithelium; the me- the highly specialized retina. Recent advances have shed soderm differentiates into the extraocular muscles and light on the interplay between numerous transcriptional the fibrous and vascular coats of the eye; and neural crest networks and growth factors that are involved in the cells become the corneal stroma sclera and corneal en- specific stages of retinogenesis, optic nerve formation, dothelium. The vertebrate eye originates from bilateral and topographic mapping. In this review, we summarize telencephalic optic grooves. In humans, optic vesicles this recent progress on the molecular mechanisms un- emerge at the end of the fourth week of development and derlying the development of the eye, visual system, and soon thereafter contact the surface ectoderm to induce embryonic tumors that arise in the optic system. -

Neuroradiology for Ophthalmologists
Eye (2020) 34:1027–1038 https://doi.org/10.1038/s41433-019-0753-z REVIEW ARTICLE Neuroradiology for ophthalmologists 1 2 1 1,3,4,5,6,7 Bayan Al Othman ● Jared Raabe ● Ashwini Kini ● Andrew G. Lee Received: 3 June 2019 / Revised: 29 October 2019 / Accepted: 24 November 2019 / Published online: 2 January 2020 © The Author(s), under exclusive licence to The Royal College of Ophthalmologists 2020 Abstract This article will review the best approaches to neuroimaging for specific ophthalmologic conditions and discuss characteristic radiographic findings. A review of the current literature was performed to find recommendations for the best approaches and characteristic radiographic findings for various ophthalmologic conditions. Options for imaging continue to grow with modern advances in technology, and ophthalmologists should stay current on the various radiographic techniques available to them, focusing on their strengths and weaknesses for different clinical scenarios. Introduction Computed tomography (CT) 1234567890();,: 1234567890();,: Modern imaging technology continues to advance the CT imaging reconstructs a three-dimensional image made boundaries and increase the options available to physicians of many conventional x-ray images. The conventional x-ray with respect to neuroradiology. In ophthalmology, the most images are ordered in tomographic slices that have been common studies employed are computed tomography (CT) computerized so a viewer can scroll through the images, and magnetic resonance imaging (MRI). There are several analyzing sequential cross-sections [1]. Density is the pri- different types of protocols that provide unique advantages mary characteristic that determines image appearance on a and disadvantages depending on the clinical scenario. This CT scan. As with traditional x-rays, tissues appear on a grey article endeavours to review those options and discuss how scale ranging from white (i.e., hyperdense tissues such as they are best employed to evaluate a variety of specific bone) to black (i.e., hypodense materials such as air). -
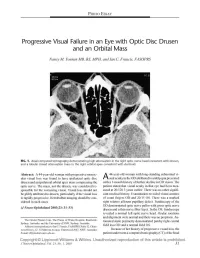
Progressive Visual Failure in an Eye with Optic Disc Drusen and an Orbital Mass
PHOTO ESSAY Progressive Visual Failure in an Eye with Optic Disc Drusen and an Orbital Mass Nancy M. Younan MB, BS, MPH, and Ian C. Francis, FASOPRS FIG. 1. Axial computed tomography demonstrating high attenuation in the right optic nerve head consistent with drusen, and a lobular mixed attenuation mass in the right orbital apex consistent with dermoid. Abstract: A 44-year-old woman with progressive monoc 44-year-old woman with long-standing subnormal vi ular visual loss was found to have ipsilateral optic disc A sual acuity in the OD attributed to amblyopia presented drusen and an ipsilateral orbital apex mass compressing the with a 1-month history of further decline in OD vision. The optic nerve. The mass, not the drusen, was considered re patient stated that visual acuity in that eye had been mea sponsible for the worsening vision. Visual loss should not sured at 20/120 3 years earlier. There was no other signifi be glibly attributed to drusen, particularly if the visual loss cant medical history. Examination revealed visual acuities is rapidly progressive. Retrobulbar imaging should be con of count fingers OD and 20/15 OS. There was a marked sidered in such cases. right relative afferent pupillary defect. Funduscopy of the OD demonstrated optic nerve pallor with gross optic nerve (JNeuro-Ophthalmol 2003;23: 31-33) drusen and a thin nerve fiber layer. In the OS, funduscopy revealed a normal left optic nerve head. Ocular rotations and alignment were normal and there was no proptosis. Au The Ocular Plastics Unit, The Prince of Wales Hospital, Randwick, tomated static perimetry demonstrated patchy right central Sydney, Australia, and the University of NSW, Sydney, Australia.