Neuroradiology for Ophthalmologists
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Unusual Presentation of Subfrontal Meningioma: a Case Report and Literature Review for Foster Kennedy Syndrome
Intern Emerg Med (2011) 6:267–269 DOI 10.1007/s11739-010-0437-y CE - MEDICAL ILLUSTRATION An unusual presentation of subfrontal meningioma: a case report and literature review for Foster Kennedy syndrome Shahram Lotfipour • Kris Chiles • J. Akiva Kahn • Tareg Bey • Scott Rudkin Received: 17 December 2009 / Accepted: 13 July 2010 / Published online: 26 August 2010 Ó SIMI 2010 Introduction head trauma. She admitted to abusing crack cocaine for 13 years with her last use 4 months ago. She denied any Foster Kennedy syndrome, named after neurologist Robert trouble with ambulation, dizziness, and changes in hearing or Foster Kennedy (1884–1952), describes unilateral ipsilat- other alterations in sensation. She denied any suicidal or eral optic atrophy and contralateral papilledema from an homicidal ideation. The patient denied any auditory halluci- intracranial mass. This syndrome is unreliably associated nations, but did report that she had been experiencing with anosmia and ipsilateral proptosis [1]. It originates visual hallucinations and visual disturbances for at least from variety of intracranial pathologies, but most often a 6–8 months. She reported complete blindness in the left eye, subfrontal mass. We present a case of Foster Kennedy and shadow perception in her right for an unknown length of syndrome and review its etiology, pathology and incidence time. Her past medical history was notable for major in intracranial tumors. depression. The patient did not have a previous history of hallucinations or psychosis, and had never been hospitalized for psychiatric reasons. The patient was not on any medica- Case report tions, and was allergic to penicillin and codeine. -

Bass – Glaucomatous-Type Field Loss Not Due to Glaucoma
Glaucoma on the Brain! Glaucomatous-Type Yes, we see lots of glaucoma Field Loss Not Due to Not every field that looks like glaucoma is due to glaucoma! Glaucoma If you misdiagnose glaucoma, you could miss other sight-threatening and life-threatening Sherry J. Bass, OD, FAAO disorders SUNY College of Optometry New York, NY Types of Glaucomatous Visual Field Defects Paracentral Defects Nasal Step Defects Arcuate and Bjerrum Defects Altitudinal Defects Peripheral Field Constriction to Tunnel Fields 1 Visual Field Defects in Very Early Glaucoma Paracentral loss Early superior/inferior temporal RNFL and rim loss: short axons Arcuate defects above or below the papillomacular bundle Arcuate field loss in the nasal field close to fixation Superotemporal notch Visual Field Defects in Early Glaucoma Nasal step More widespread RNFL loss and rim loss in the inferior or superior temporal rim tissue : longer axons Loss stops abruptly at the horizontal raphae “Step” pattern 2 Visual Field Defects in Moderate Glaucoma Arcuate scotoma- Bjerrum scotoma Focal notches in the inferior and/or superior rim tissue that reach the edge of the disc Denser field defects Follow an arcuate pattern connected to the blind spot 3 Visual Field Defects in Advanced Glaucoma End-Stage Glaucoma Dense Altitudinal Loss Progressive loss of superior or inferior rim tissue Non-Glaucomatous Etiology of End-Stage Glaucoma Paracentral Field Loss Peripheral constriction Hereditary macular Loss of temporal rim tissue diseases Temporal “islands” Stargardt’s macular due -

Bilateral Acquired Progressive Retinal Nerve Fiber Layer Myelination
Bilateral Acquired Progressive Retinal Nerve Fiber Layer Myelination Abstract We present the multimodal imaging findings of an unusual case of bilateral acquired progressive myelination of the optic disc over a 10-year follow up period, in a hyperopic adolescent patient in the absence of an underlying ocular or systemic abnormality. Myelination of the left optic disc was noted at age 7 and of the right optic disc at age 13 but no other ocular or systemic abnormalities were identified. Cross sectional OCT and en face OCT angiography confirmed the presence of myelination of the retinal nerve fiber layer and excluded other etiologic possibilities including an astrocytic hamartoma. Keywords: Optic Nerve; Optic Fiber Myelination, Acquired, Hyperopia INTRODUCTION Myelination of the retinal nerve fiber layer occurs in 1% of the population and is most frequently congenital and non-progressive. (1) Congenital cases are typically isolated but may be rarely associated with the syndrome of ipsilateral high myopia, strabismus and amblyopia (2,3), although cases have been reported in hyperopic eyes. Reports of acquired and progressive myelination associated with congenital optic nerve disorders such as Arnold-Chiari malformation, hydrocephalus, optic disc drusen, and iatrogenic causes such as optic nerve sheath fenestration, are relatively rare.(4–8) PLEASE ADJUST THE REFERENCE CITATIONS While affected patients are typically asymptomatic, retinal nerve fiber layer myelination can be rarely associated with visual loss. Even more unusual are reports of acquired and progressive myelination of the nerve fiber layer. We present the multimodal imaging findings of such a case in a healthy young patient with a 10-year follow up. -

Annual Report Research Activity 2019
Annual Report Research Activity 2019 Division of Clinical Neuroscience University of Oslo and Oslo University Hospital 0 Contents Oslo University Hospital and the University of Oslo .................................................................................... 4 From Division Director Eva Bjørstad ........................................................................................................... 4 Division of Clinical Neuroscience (NVR) Organizational Chart ..................................................................... 5 Department of Physical Medicine and Rehabilitation Rehabilitation after trauma....................................................................................................................... 6 Group Leader: Nada Andelic Painful musculoskeletal disorders .............................................................................................................. 9 Group Leader: Cecilie Røe Department of Refractory Epilepsy - National Centre for Epilepsy Complex epilepsy .................................................................................................................................... 11 Group Leader: Morten Lossius Department of Neurosurgery Neurovascular-Hydrocephalus Research Group ..................................................................................... 16 Group Leader: Per Kristian Eide Oslo Neurosurgical Outcome Study Group (ONOSG) ................................................................................. 19 Group Leaders: Eirik Helseth and Torstein -

References Briskly Compared with the Fellow Eye
LETTERS TO THE JOURNAL 367 frontal tumour. The optic atrophy is commonly felt to Sir, result from optic nerve compression and the contralateral Apraclonidine in the Management of Glaucomatocy 1.2 papilloedema from increased intracranial pressure. clitic crisis Another mechanism suggests that Foster Kennedy syn Glaucomatocyclitic cnSlS (Posner-Schlossman syn drome is due to bilateral direct optic nerve compression by drome) is a unilateral inflammation of the uveal tract in a midline basal mass or less commonly by long-standing which signs of an acute increase in intraocular pressure increased intracranial pressure without direct com predominate. As the aetiology is doubtful, numerous treat pression of either nerve.3 ments have been suggested, the main aim being to reduce Since the early cases of Foster Kennedy syndrome, the exceptionally high intraocular pressure which, left many cases have been reported in the literature caused by untreated, will cause permanent optic nerve damage. other tumours, especially meningiomas such as olfactory Apraclonidine hydrochloride I %, a clonidine deriva groove and sphenoid ridge meningiomas, with gliomas tive and a peripheral alpha-adrenergic agonist. was devel occasionally reported.�-7 To our knowledge, nasopharyn oped to lower intraocular pressure while minimising geal carcinoma is rarely reported in the literature as a systemic side effects. It has specificrecept or-binding and cause of Foster Kennedy syndrome. physico chemical properties that limit its access to the cen Other terms have been used in the literature to describe tral nervous system. In normal human volunteers it pro atypical cases of Foster Kennedy syndrome. 'Pseudo Fos duces a significant fall in intraocular pressure. -

Central Serous Papillopathy by Optic Nerve Head Drusen
Clinical Ophthalmology Dovepress open access to scientific and medical research Open Access Full Text Article CASE REPORT Central serous papillopathy by optic nerve head drusen Ana Marina Suelves1 Abstract: We report a 38-year-old man with a complaint of blurred vision in his right eye for the Ester Francés-Muñoz1 previous 5 days. He had bilateral optic disc drusen. Fluorescein angiography revealed multiple Roberto Gallego-Pinazo1 hyperfluorescent foci within temporal optic discs and temporal inferior arcade in late phase. Diamar Pardo-Lopez1 Optical coherence tomography showed bilateral peripapillary serous detachment as well as right Jose Luis Mullor2 macular detachment. This is the first reported case of a concurrent peripapillary and macular Jose Fernando Arevalo3 detachment in a patient with central serous papillopathy by optic disc drusen. Central serous papillopathy is an atypical form of central serous chorioretinopathy that should be considered Manuel Díaz-Llopis1,4,5 as a potential cause of acute loss of vision in patients with optic nerve head drusen. 1 Department of Ophthalmology, La Fe Keywords: central serous papillopathy, peripapillary central serous chorioretinopathy, optic University Hospital, Valencia, Spain; For personal use only. 2Instituto de Investigación Sanitaria, nerve head drusen, peripapillary subretinal fluid Fundación para la investigación, La Fe Hospital, Valencia, Spain; 3Retina and vitreous service, Clínica Introduction Oftalmológica Centro Caracas, Optic nerve head drusen (ONHD) are hyaline material calcificated -

Fluorescein Angiography Reference Number: OC.UM.CP.0028 Coding Implications Last Review Date: 05/2020 Revision Log
Clinical Policy: Fluorescein Angiography Reference Number: OC.UM.CP.0028 Coding Implications Last Review Date: 05/2020 Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description Intravenous Fluorescein Angiography (IVFA) or fluorescent angiography is a technique for examining the circulation of the retina and choroid using a fluorescent dye and a specialized camera. It involves injection of sodium fluorescein into the systemic circulation, and then an angiogram is obtained by photographing the fluorescence emitted after illumination of the retina with blue light at a wavelength of 490 nanometers. This policy describes the medical necessity guidelines for fluorescein angiography. Policy/Criteria I. It is the policy of health plans affiliated with Envolve Vision, Inc.® that fluorescein angiography is medically necessary for the following indications: A. Initial evaluation of a patient with abnormal findings of the fundus / retina on ophthalmoscopy exam including one of the following: 1. Choroidal Neovascular Membranes (CNVM) 2. Lesions of the Retinal Pigment Epithelium (RPE) a. Serous Detachment of the RPE b. Tears or rips of the RPE c. Hemorrhagic detachment 3. Fibrovascular Disciform Scar 4. Vitreous Hemorrhage (patient presents with sudden loss of vision) 5. Drusen 6. Diabetic Retinopathy B. Evaluation of patient presenting with symptoms of sudden vision loss (especially central vision), blurred vision, distortion, etc., which may suggest a subretinal neovascularization and abnormal findings of the fundus / retina on ophthalmoscopy exam. C. Evaluation of patients with nonproliferative (background) and proliferative diabetic retinopathy without macular edema. Frequency is determined by disease progression and the treatment performed. Fluorescein angiography may be performed on the treated eye only at 6 weeks post-treatment and as often as every 8-12 weeks to assist in management of the retinopathy. -

A Case of Foster Kennedy Syndrome in a Pregnant Lady Presenting with Unilateral Deterioration of Vision
Mehmood A, et al., J Ophthalmic Clin Res 2021, 8: 076 DOI: 10.24966/OCR-8887/100076 HSOA Journal of Ophthalmology & Clinical Research Case Report last 4 months but over the last week before presentation, it had fallen A Case of Foster Kennedy precipitously to counting fingers. The vision in her right eye has also deteriorated over the preceding week together with frontal headaches Syndrome in a Pregnant Lady but not associated with nausea and vomiting. On examination, she was generally well, afebrile, alert, and oriented. Visual acuities were Presenting with Unilateral 6/18 in her right eye and counting fingers in the left eye not improving with pinhole or refraction. On slit lamp, anterior segment examination Deterioration of Vision of both eyes was unremarkable and fundal examination revealed Asif Mehmood1, Farooq Ul Abidin2* and Sharjeel Khan3 marked papilloedema on the right eye and optic atrophy on the left eye (Figure 1A,1B). There was no relative afferent pupillary defect, 1Consultant Ophthalmologist, Rehman medical institute, Peshawar, Pakistan no proptosis, no bruit, and extraocular movements of both the eyes 2Resident Ophthalmology, AFIO, Rawalpindi, Pakistan were full range. There were no other focal or generalized neurological 3Department of Ophthalmology, Dera Ismail Khan, Pakistan signs. Abstract Foster Kennedy syndrome is a rare neurological entity that includes ipsilateral optic atrophy, contralateral papilledema, and sometimes anosmia. The syndrome has been described in association with a variety of intracranial pathologies such as a large frontal lobe tumor, olfactory groove meningioma, or medial third sphenoidal wing meningioma. In this report, we present a case of sphenoidal wing meningioma with Foster Kennedy syndrome in a 25-year-old pregnant female. -

Astrocytic Tumors
Neuro-ophthalmologic Examination of the Neurosurgical Patients Amgad Hanna, MD and Peter Savino, MD Departments of Neurosurgery and Ophthalmology Thomas Jefferson University December 2005 Agenda • Abnormal pupils: miosis and mydriasis • Trochlear N palsy • Fundi: NL and abnormal Abnormal pupils Miosis Not all constricted pupils are caused by carotid dissection V1 Postganglionic (3rd order) Horner’s Long Cil Nn Central (1st order) Horner’s Preganglionic (2nd order) Horner’s Causes of Horner’s Syndrome (usually have associated signs and symptoms) Cocaine NO Mydriasis response NE NE Blocks reuptake NE NE Of NE (90%) NO NE outside vesicles True Pseudo-Horner’s Horner’s (physiologic anisocoria) (MRI/A H/N – CT Chest) True Horner’s Hydroxyamphetamine test No response Mydriasis NE NE Release of NE from No NE NE NE inside vesicles synaptic vesicles NE Postganglionic Central or Horner’s Preganglionic Horner’s Physiologic Anisocoria Central or Preganglionic R Horner’s Postganglionic R Horner’s Case • 40 y/o Woman • C/O R swollen eyelid and H/A Upper lid (Muller’s m), lower lid (Lid retractis) Dim Light Post-Cocaine; True Horner’s Post-Hydroxyamphetamine; Postganglionic Horner’s Carotid Dissection Mydriasis Not all dilated pupils are caused by P Com aneurysms Parasympathetic and light reflex Posterior commissure III Inf div Short Cil Nn N to inf obl Physiologic anisocoria • Normal reaction to light • The size difference between both eyes remains the same with dim and bright light • 0.3 – 0.4 mm difference found in 50% of the normal population • Up -
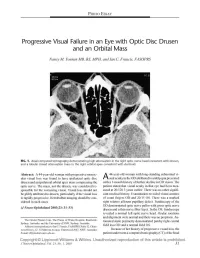
Progressive Visual Failure in an Eye with Optic Disc Drusen and an Orbital Mass
PHOTO ESSAY Progressive Visual Failure in an Eye with Optic Disc Drusen and an Orbital Mass Nancy M. Younan MB, BS, MPH, and Ian C. Francis, FASOPRS FIG. 1. Axial computed tomography demonstrating high attenuation in the right optic nerve head consistent with drusen, and a lobular mixed attenuation mass in the right orbital apex consistent with dermoid. Abstract: A 44-year-old woman with progressive monoc 44-year-old woman with long-standing subnormal vi ular visual loss was found to have ipsilateral optic disc A sual acuity in the OD attributed to amblyopia presented drusen and an ipsilateral orbital apex mass compressing the with a 1-month history of further decline in OD vision. The optic nerve. The mass, not the drusen, was considered re patient stated that visual acuity in that eye had been mea sponsible for the worsening vision. Visual loss should not sured at 20/120 3 years earlier. There was no other signifi be glibly attributed to drusen, particularly if the visual loss cant medical history. Examination revealed visual acuities is rapidly progressive. Retrobulbar imaging should be con of count fingers OD and 20/15 OS. There was a marked sidered in such cases. right relative afferent pupillary defect. Funduscopy of the OD demonstrated optic nerve pallor with gross optic nerve (JNeuro-Ophthalmol 2003;23: 31-33) drusen and a thin nerve fiber layer. In the OS, funduscopy revealed a normal left optic nerve head. Ocular rotations and alignment were normal and there was no proptosis. Au The Ocular Plastics Unit, The Prince of Wales Hospital, Randwick, tomated static perimetry demonstrated patchy right central Sydney, Australia, and the University of NSW, Sydney, Australia. -

Optic Disc Drusen, Glaucoma, Or Could It Be Both?
Title: Where are the defects coming from…Optic Disc Drusen, Glaucoma, or could it be both? Authors: Rachel Goretsky OD, Biana Gekht OD Abstract: Optic disc drusen and glaucoma cause similar retinal nerve fiber layer(RNFL) as well as visual field defects. This paper describes a patient who has both conditions and the management involved. I. Case History: 66 year old African-American male presents for dilation and imaging. Patient has a history of Glaucoma for several years; he is taking Brimonidine bid OU and Latanoprost qhs OU with reported good compliance. Medical & Ocular History: • Hypertension, gout, cholesterol and stents placed in leg and chest. Glaucoma OU. History of peripheral iridotomy OU one year ago. Medications:Amplodipine, clopidogrel, allopurinol II. Pertinent findings: • Maximum pressure history is 17 OD, 19 OS, current visit 16 OD, 19 OS • Anterior chamber : Grade 2 Van Herick Angles OU • Iris: Peripheral Iridotomy, patent at 12 o’clock OD,OS • Optic disc: 0.3r OD, OS small nerves, mild blurry margins indicative of disc drusen • Macula: diffuse epiretinal membrane OU • Pachymetry : 556 OD/ 566 OS • Gonioscopy: open to trabecular meshwork (TM) superiorly, otherwise no structures visible OD, open to TM inferiorly, otherwise no structures visible OS • Spectralis Optical Coherence Tomography(OCT): optic disc drusen with small cups and disc area OU • Visual field exhibited no defects OD, inferior nasal defects OS. III. Differential Diagnosis: • Papilledema, tilted discs, pseudotumor cerebri, myelinated nerve fiber layer IV. Diagnosis and Discussion Optic nerve head drusen are hyaline deposits made of calcium phosphate as well as amino acids, among other materials. -
Optic Nerve on Sheath:
3/16/2018 < Optic nerve axons of retinal ganglion cells 1.2 million nerve fibers . ON sheath: continuous with the meninges dura、arachnoid and pia mater 1 3/16/2018 optic nerve functions 1.Visual Acuity 2.Color Vision 3.Pupil 4.Contrast sensitivity Ancillary Tests 1.Visual Field 2.Neuro-imaging 3.OCT 4.VEP Etiology:Optic nerve diseases 1.inflammation:optic neuritis 2 . ischemic optic neuropathy 3-Compression 4-Granuloma & infiltration 5-Hereditary 6-Toxic 7-Irradiation 8- Trauma . 2 3/16/2018 Optic Neuritis 3-Neuroretinitis 1-Retrobulbar neuritis 2-Papillitis Papillitis optic disc is normal hyperemia and edema with macular star . in adults common in children. least common type with multiple sclerosis. viral infections Rapid unilateral loss of vision RAPD Loss of color vision Pain in moving the eye Swollen disc with or without peripapillary flame-shaped hemorrhages. 3 3/16/2018 Fig optic neuritis Centrocecal scotoma Bilateral optic neuritis Bilateral Central scotoma 4 3/16/2018 Bilateral hemianopsia MRI demyelinating lesions multiple sclerosis Neuromylitis Optica VF showed non- central scotoma altitudinal VF an ischemic mechanism play a role in ON in NMO patients 5 3/16/2018 Optic Neuritis Follow Up Diffuse and central loss in the affected eye at baseline Follow Up : nerve fiber bundle defects were the predominant localized abnormalities in both the affected and fellow eyes physicians evaluate the characteristics of optic neuritis and other optic neuropathies in the future Ischemic optic neuropathy ( ION) 6 3/16/2018 Anterior ischemic optic neuropathy ( AION ) Arteritic Non- Arteritic GCA - vasculitis hypoperfusion of ONH transient visual loss, temporal sudden painless loss of vision pain, jaw pain, fatigue, weight loss hyperemic disc Pale disc Sectorial, diffuse edema optic disc edema splinter hemorrhages of a chalky white color Subsequent optic atrophy Nonarteritic ischemic optic neuropathy visual field altitudinal field defect 7 3/16/2018 NAION sudden, painless visual loss OS shows AION .