Analysis of the Active Site Cysteine Residue of the Sacrificial Sulfur
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Aryl Hydrocarbon Receptor: Structural Analysis and Activation Mechanisms
The Aryl Hydrocarbon Receptor: Structural Analysis and Activation Mechanisms This thesis is submitted in fulfilment of the requirements for the degree of Doctor of Philosophy in the School of Molecular and Biomedical Sciences (Biochemistry), The University of Adelaide, Australia Fiona Whelan, B.Sc. (Hons) 2009 2 Table of Contents THESIS SUMMARY................................................................................. 6 DECLARATION....................................................................................... 7 PUBLICATIONS ARISING FROM THIS THESIS.................................... 8 ACKNOWLEDGEMENTS...................................................................... 10 ABBREVIATIONS ................................................................................. 12 CHAPTER 1: INTRODUCTION ............................................................. 17 1.1 BHLH.PAS PROTEINS ............................................................................................17 1.1.1 General background..................................................................................17 1.1.2 bHLH.PAS Class I Proteins.........................................................................18 1.2 THE ARYL HYDROCARBON RECEPTOR......................................................................19 1.2.1 Domain Structure and Ligand Activation ..............................................19 1.2.2 AhR Expression and Developmental Activity .......................................21 1.2.3 Mouse AhR Knockout Phenotype ...........................................................23 -

Non-Homologous Isofunctional Enzymes: a Systematic Analysis Of
Omelchenko et al. Biology Direct 2010, 5:31 http://www.biology-direct.com/content/5/1/31 RESEARCH Open Access Non-homologousResearch isofunctional enzymes: A systematic analysis of alternative solutions in enzyme evolution Marina V Omelchenko, Michael Y Galperin*, Yuri I Wolf and Eugene V Koonin Abstract Background: Evolutionarily unrelated proteins that catalyze the same biochemical reactions are often referred to as analogous - as opposed to homologous - enzymes. The existence of numerous alternative, non-homologous enzyme isoforms presents an interesting evolutionary problem; it also complicates genome-based reconstruction of the metabolic pathways in a variety of organisms. In 1998, a systematic search for analogous enzymes resulted in the identification of 105 Enzyme Commission (EC) numbers that included two or more proteins without detectable sequence similarity to each other, including 34 EC nodes where proteins were known (or predicted) to have distinct structural folds, indicating independent evolutionary origins. In the past 12 years, many putative non-homologous isofunctional enzymes were identified in newly sequenced genomes. In addition, efforts in structural genomics resulted in a vastly improved structural coverage of proteomes, providing for definitive assessment of (non)homologous relationships between proteins. Results: We report the results of a comprehensive search for non-homologous isofunctional enzymes (NISE) that yielded 185 EC nodes with two or more experimentally characterized - or predicted - structurally unrelated proteins. Of these NISE sets, only 74 were from the original 1998 list. Structural assignments of the NISE show over-representation of proteins with the TIM barrel fold and the nucleotide-binding Rossmann fold. From the functional perspective, the set of NISE is enriched in hydrolases, particularly carbohydrate hydrolases, and in enzymes involved in defense against oxidative stress. -

Biosynthesis in Vitro of Bacillamide Intermediate-Heterocyclic Alacysthiazole by Heterologous Expression of Nonribosomal Peptide Synthetase (NRPS) T
Journal of Biotechnology 292 (2019) 5–11 Contents lists available at ScienceDirect Journal of Biotechnology journal homepage: www.elsevier.com/locate/jbiotec Biosynthesis in vitro of bacillamide intermediate-heterocyclic AlaCysthiazole by heterologous expression of nonribosomal peptide synthetase (NRPS) T Fengli Zhang, Nayila Mulati, Yukun Wang, Yingxin Li, Sanqiang Gong, Loganathan Karthik, ⁎ Wei Sun, Zhiyong Li Marine Biotechnology Laboratory, State Key Laboratory of Microbial Metabolism and School of Life Sciences & Biotechnology, Shanghai Jiao Tong University, Shanghai, China ARTICLE INFO ABSTRACT Keywords: Bacillamide C, a potential natural antialgae active compound, is produced by Bacillus atrophaeus C89 derived Bacillus atrophaeus from marine sponge Dysidea avara. A nonribosomal peptide synthetase (NRPS) cluster is hypothesized to be Bacillamides involved in the biosynthesis of bacillamide C. The NRPS with a domain string of A1-PCP1-Cy-A2-PCP2-C can be Heterologous expression divided into three functional modules. After heterologous expression and purification of module A1-PCP1 and Nonribosomal peptide synthetase (NRPS) module Cy-A2-PCP2, their catalytic activities were biochemically proven in vitro by the reaction with the apo- Thiazole PCP domain transformed to the holo-PCP domain through a phosphopantetheinyl transferase, ATP, and substrate amino acids. Five– membered heterocyclic AlaCysthiazole with molecular weight of 172.0389 was detected. This proved the formation of the heterocyclic dipeptide AlaCysthiazole, which is considered to be a building block for the biosynthesis of bacillamide. This study provides a basis for further biosynthesis of bacillamides. 1. Introduction et al., 2017). Even though the biosynthesis of bacillamide C was opti- mized, the yield was very low (Jin et al., 2011; Yu et al., 2015). -

The Microbiota-Produced N-Formyl Peptide Fmlf Promotes Obesity-Induced Glucose
Page 1 of 230 Diabetes Title: The microbiota-produced N-formyl peptide fMLF promotes obesity-induced glucose intolerance Joshua Wollam1, Matthew Riopel1, Yong-Jiang Xu1,2, Andrew M. F. Johnson1, Jachelle M. Ofrecio1, Wei Ying1, Dalila El Ouarrat1, Luisa S. Chan3, Andrew W. Han3, Nadir A. Mahmood3, Caitlin N. Ryan3, Yun Sok Lee1, Jeramie D. Watrous1,2, Mahendra D. Chordia4, Dongfeng Pan4, Mohit Jain1,2, Jerrold M. Olefsky1 * Affiliations: 1 Division of Endocrinology & Metabolism, Department of Medicine, University of California, San Diego, La Jolla, California, USA. 2 Department of Pharmacology, University of California, San Diego, La Jolla, California, USA. 3 Second Genome, Inc., South San Francisco, California, USA. 4 Department of Radiology and Medical Imaging, University of Virginia, Charlottesville, VA, USA. * Correspondence to: 858-534-2230, [email protected] Word Count: 4749 Figures: 6 Supplemental Figures: 11 Supplemental Tables: 5 1 Diabetes Publish Ahead of Print, published online April 22, 2019 Diabetes Page 2 of 230 ABSTRACT The composition of the gastrointestinal (GI) microbiota and associated metabolites changes dramatically with diet and the development of obesity. Although many correlations have been described, specific mechanistic links between these changes and glucose homeostasis remain to be defined. Here we show that blood and intestinal levels of the microbiota-produced N-formyl peptide, formyl-methionyl-leucyl-phenylalanine (fMLF), are elevated in high fat diet (HFD)- induced obese mice. Genetic or pharmacological inhibition of the N-formyl peptide receptor Fpr1 leads to increased insulin levels and improved glucose tolerance, dependent upon glucagon- like peptide-1 (GLP-1). Obese Fpr1-knockout (Fpr1-KO) mice also display an altered microbiome, exemplifying the dynamic relationship between host metabolism and microbiota. -

Harmonization Project Document No. 4
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the World Health Organization, the International Labour Organization, or the United Nations Environment Programme. Harmonization Project Document No. 4 PART 1: IPCS FRAMEWORK FOR ANALYSING THE RELEVANCE OF A CANCER MODE OF ACTION FOR HUMANS AND CASE-STUDIES PART 2: IPCS FRAMEWORK FOR ANALYSING THE RELEVANCE OF A NON-CANCER MODE OF ACTION FOR HUMANS This project was conducted within the IPCS project on the Harmonization of Approaches to the Assessment of Risk from Exposure to Chemicals. Published under the joint sponsorship of the World Health Organization, the International Labour Organization, and the United Nations Environment Programme, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals. The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organization (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals. The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and Agriculture Organization of the United Nations, WHO, the United Nations Industrial Development Organization, the United Nations Institute for Training and Research, and the Organisation for Economic Co-operation and Development (Participating Organizations), following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase coordination in the field of chemical safety. -
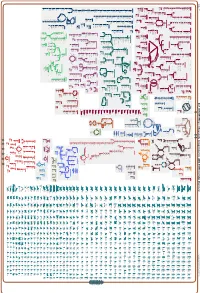
Generate Metabolic Map Poster
Authors: Pallavi Subhraveti Ron Caspi Peter Midford Peter D Karp An online version of this diagram is available at BioCyc.org. Biosynthetic pathways are positioned in the left of the cytoplasm, degradative pathways on the right, and reactions not assigned to any pathway are in the far right of the cytoplasm. Transporters and membrane proteins are shown on the membrane. Ingrid Keseler Periplasmic (where appropriate) and extracellular reactions and proteins may also be shown. Pathways are colored according to their cellular function. Gcf_003855395Cyc: Shewanella livingstonensis LMG 19866 Cellular Overview Connections between pathways are omitted for legibility. -

Auxiliary Table 2 Proteomics.Xlsx
Gene names log2(fold-Change) -Log(p-value) Significant Protein IDs Majority protein IDs Protein names gatZ -3.515 2.827 + P0C8J8;P0C8K0 P0C8J8 D-tagatose-1,6-bisphosphate aldolase subunit GatZ dps -3.362 4.596 + P0ABT2 P0ABT2 DNA protection during starvation protein sstT -3.219 3.423 + P0AGE4 P0AGE4 Ser/Thr transporter SstT iraP -3.074 3.623 + P0AAN9 P0AAN9 Anti-adapter protein IraP ilvC -3.054 3.692 + P05793 P05793 Ketol-acid reductoisomerase rfbB -3.006 4.290 + P37759 P37759 dTDP-glucose 4,6-dehydratase 1 gdhA -2.847 5.732 + P00370 P00370 NADP-specific glutamate dehydrogenase rstA -2.796 3.542 + P52108 P52108 Transcriptional regulatory protein RstA ompT -2.790 4.251 + P09169 P09169 Protease 7 uspF -2.719 4.817 + P37903 P37903 Universal stress protein F crl -2.649 4.248 + P24251 P24251 σ factor-binding protein Crl yncE -2.514 1.994 + P76116 P76116 Uncharacterized protein YncE yiiS -2.507 4.223 + P32162 P32162 UPF0381 protein YiiS yagU -2.494 2.430 + P0AAA1 P0AAA1 Inner membrane protein YagU stpA -2.488 3.540 + P0ACG1 P0ACG1 DNA-binding protein StpA ydgJ -2.476 3.366 + P77376 P77376 Uncharacterized oxidoreductase YdgJ kgtP -2.462 2.665 + P0AEX3 P0AEX3 α-ketoglutarate permease sodB -2.436 3.704 + P0AGD3 P0AGD3 Superoxide dismutase [Fe] purT -2.386 2.851 + P33221 P33221 Phosphoribosylglycinamide formyltransferase 2 trpA -2.371 3.261 + P0A877 P0A877 Trp synthase α chain acnB -2.319 2.707 + P36683 P36683 Aconitate hydratase B iadA -2.316 4.127 + P39377 P39377 Isoaspartyl dipeptidase modF -2.244 2.107 + P31060 P31060 Putative molybdenum transport -
![The [4Fe-4S] Cluster of Sulfurtransferase Ttua Desulfurizes](https://docslib.b-cdn.net/cover/2676/the-4fe-4s-cluster-of-sulfurtransferase-ttua-desulfurizes-2862676.webp)
The [4Fe-4S] Cluster of Sulfurtransferase Ttua Desulfurizes
ARTICLE https://doi.org/10.1038/s42003-020-0895-3 OPEN The [4Fe-4S] cluster of sulfurtransferase TtuA desulfurizes TtuB during tRNA modification in Thermus thermophilus ✉ Minghao Chen 1,7, Masato Ishizaka2,7, Shun Narai2,7, Masaki Horitani 3, Naoki Shigi 4, Min Yao1 & ✉ 1234567890():,; Yoshikazu Tanaka 1,5,6 TtuA and TtuB are the sulfurtransferase and sulfur donor proteins, respectively, for bio- synthesis of 2-thioribothymidine (s2T) at position 54 of transfer RNA (tRNA), which is responsible for adaptation to high temperature environments in Thermus thermophilus. The enzymatic activity of TtuA requires an iron-sulfur (Fe-S) cluster, by which a sulfur atom supplied by TtuB is transferred to the tRNA substrate. Here, we demonstrate that the Fe-S cluster directly receives sulfur from TtuB through its inherent coordination ability. TtuB forms a [4Fe-4S]-TtuB intermediate, but that sulfur is not immediately released from TtuB. Further desulfurization assays and mutation studies demonstrated that the release of sulfur from the thiocarboxylated C-terminus of TtuB is dependent on adenylation of the substrate tRNA, and the essential residue for TtuB desulfurization was identified. Based on these findings, the molecular mechanism of sulfur transfer from TtuB to Fe-S cluster is proposed. 1 Faculty of Advanced Life Science, Hokkaido University, Kita 8, Nishi 5, Kita-ku, Sapporo, Hokkaido 060-0810, Japan. 2 Graduate School of Life Science, Hokkaido University, Kita 8, Nishi 5, Kita-ku, Sapporo, Hokkaido 060-0810, Japan. 3 Faculty of Agriculture, Department of Applied Biochemistry and Food Science, Saga University, 1 Honjo-machi, Saga 840-8502, Japan. 4 Biotechnology Research Institute for Drug Discovery, National Institute of Advanced Industrial Science and Technology, 2-4-7 Aomi, Koto-ku, Tokyo 135-0064, Japan. -

Introduction (Pdf)
Dictionary of Natural Products on CD-ROM This introduction screen gives access to (a) a general introduction to the scope and content of DNP on CD-ROM, followed by (b) an extensive review of the different types of natural product and the way in which they are organised and categorised in DNP. You may access the section of your choice by clicking on the appropriate line below, or you may scroll through the text forwards or backwards from any point. Introduction to the DNP database page 3 Data presentation and organisation 3 Derivatives and variants 3 Chemical names and synonyms 4 CAS Registry Numbers 6 Diagrams 7 Stereochemical conventions 7 Molecular formula and molecular weight 8 Source 9 Importance/use 9 Type of Compound 9 Physical Data 9 Hazard and toxicity information 10 Bibliographic References 11 Journal abbreviations 12 Entry under review 12 Description of Natural Product Structures 13 Aliphatic natural products 15 Semiochemicals 15 Lipids 22 Polyketides 29 Carbohydrates 35 Oxygen heterocycles 44 Simple aromatic natural products 45 Benzofuranoids 48 Benzopyranoids 49 1 Flavonoids page 51 Tannins 60 Lignans 64 Polycyclic aromatic natural products 68 Terpenoids 72 Monoterpenoids 73 Sesquiterpenoids 77 Diterpenoids 101 Sesterterpenoids 118 Triterpenoids 121 Tetraterpenoids 131 Miscellaneous terpenoids 133 Meroterpenoids 133 Steroids 135 The sterols 140 Aminoacids and peptides 148 Aminoacids 148 Peptides 150 β-Lactams 151 Glycopeptides 153 Alkaloids 154 Alkaloids derived from ornithine 154 Alkaloids derived from lysine 156 Alkaloids -

Conserved Active Site Cysteine Residue of Archaeal THI4 Homolog Is Essential for Thiamine Biosynthesis in Haloferax Volcanii Hwang Et Al
glycine COOH CONH 2 H2N COOH COOH + NUDIX N N ADP-O S hydrolase? N S O THI4-SH THI4-C=CH ? OH OH O-ADP OP HVO_0665 ADT S NAD HET-P or OP THZ-P + ThiE, ThiN N N THI6-Ntd N TMP HVO_2668 NH2 HVO_0662 ATP ThiL N ThiD HVO_1861 THI20-/THI21-Ntd Purine N ThiC PurM O NH2 N ATP N S biosynthetic SAM OPP PO + pathway HVO_1557 HVO_2154 N OP HVO_2666 N OPP N N OHOH NH2 NH2 AIR HMP-P HMP-PP N TPP NH2 Conserved active site cysteine residue of archaeal THI4 homolog is essential for thiamine biosynthesis in Haloferax volcanii Hwang et al. Hwang et al. BMC Microbiology 2014, 14:260 http://www.biomedcentral.com/1471-2180/14/260 Hwang et al. BMC Microbiology 2014, 14:260 http://www.biomedcentral.com/1471-2180/14/260 RESEARCH ARTICLE Open Access Conserved active site cysteine residue of archaeal THI4 homolog is essential for thiamine biosynthesis in Haloferax volcanii Sungmin Hwang1, Bryan Cordova1, Nikita Chavarria1, Dina Elbanna1, Stephen McHugh1, Jenny Rojas1, Friedhelm Pfeiffer3 and Julie A Maupin-Furlow1,2* Abstract Background: Thiamine (vitamin B1) is synthesized de novo by certain yeast, fungi, plants, protozoans, bacteria and archaea. The pathway of thiamine biosynthesis by archaea is poorly understood, particularly the route of sulfur relay to form the thiazole ring. Archaea harbor structural homologs of both the bacterial (ThiS-ThiF) and eukaryotic (THI4) proteins that mobilize sulfur to thiazole ring precursors by distinct mechanisms. Results: Based on comparative genome analysis, halophilic archaea are predicted to synthesize the pyrimidine moiety of thiamine by the bacterial pathway, initially suggesting that also a bacterial ThiS-ThiF type mechanism for synthesis of the thiazole ring is used in which the sulfur carrier ThiS is first activated by ThiF-catalyzed adenylation. -

Genetic and Biochemical Characterization of Yrkf, a Novel
Genetic and biochemical characterization of YrkF, a novel two-domain sulfurtransferase in Bacillus subtilis Jeremy Hunt Thesis submitted to the faculty of Virginia Polytechnic Institute and State University in partial fulfillment of the requirements for the degree of Master of Science In Biochemistry Timothy J. Larson, Chair Dennis R. Dean Brenda J. Winkel August 11, 2004 Blacksburg, Virginia Keywords: YrkF, rhodanese, Ccd1, persulfide sulfur, disulfide cross-linking Genetic and biochemical characterization of YrkF, a novel two-domain sulfurtransferase in Bacillus subtilis Jeremy Hunt Timothy J. Larson, Chair Department of Biochemistry ABSTRACT Sulfur-containing compounds such as thiamin, biotin, molybdopterin, lipoic acid, and [Fe-S] clusters are essential for life. Sulfurtransferases are present in eukaryotes, eubacteria, and archaea and are believed to play important roles in mobilizing sulfur necessary for biosynthesis of these compounds and for normal cellular functions. The rhodanese homology domain is a ubiquitous structural module containing a characteristic active site cysteine residue. Some proteins containing a rhodanese domain display thiosulfate:cyanide sulfurtransferase activity in vitro. However, the physiological functions of rhodaneses remain largely unknown. YrkF, the first rhodanese to be characterized from Bacillus subtilis, is a unique protein containing two domains, an N-terminal Ccd1 domain and a C-terminal rhodanese domain. Ccd1 (conserved cysteine domain 1) is a ubiquitous structural module characterized by a Cys-Pro-X-Pro sequence motif. Thus, YrkF contains two cysteine residues (Cys15 and Cys149), one in each domain. Biochemical, genetic, and bioinformatic approaches were used in order to characterize YrkF. First, YrkF was overexpressed and assayed for rhodanese activity to show that the protein is a functional rhodanese. -

Sex-Dependent and -Independent Transcriptional Changes
bioRxiv preprint doi: https://doi.org/10.1101/690289; this version posted July 2, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Sex-dependent and -independent transcriptional changes during 2 haploid phase gametogenesis in the sugar kelp 3 Saccharina latissima 4 5 6 7 8 Gareth A Pearson1*¶, Neusa Martins1¶, Pedro Madeira1, Ester A Serrão1, Inka Bartsch2 9 10 1Centre for Marine Sciences (CCMAR)-CIMAR, University of Algarve, Faro 8005-139, Portugal 11 2 Alfred-Wegener-Institute, Helmholtz Center for Polar and Marine Research, Am Handelshafen 12, 12 27570 Bremerhaven, Germany 13 14 15 16 * Corresponding author 17 E-mail: [email protected] 18 19 ¶These authors contributed equally to this work 20 21 1 bioRxiv preprint doi: https://doi.org/10.1101/690289; this version posted July 2, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 22 Abstract 23 In haplodiplontic lineages, sexual reproduction occurs in haploid parents without meiosis. Although 24 widespread in multicellular lineages such as brown algae (Phaeophyceae), haplodiplontic 25 gametogenesis has been little studied at the molecular level. We addressed this by generating an 26 annotated reference transcriptome for the gametophytic phase of the sugar kelp, Saccharina 27 latissima.