The [4Fe-4S] Cluster of Sulfurtransferase Ttua Desulfurizes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Aryl Hydrocarbon Receptor: Structural Analysis and Activation Mechanisms
The Aryl Hydrocarbon Receptor: Structural Analysis and Activation Mechanisms This thesis is submitted in fulfilment of the requirements for the degree of Doctor of Philosophy in the School of Molecular and Biomedical Sciences (Biochemistry), The University of Adelaide, Australia Fiona Whelan, B.Sc. (Hons) 2009 2 Table of Contents THESIS SUMMARY................................................................................. 6 DECLARATION....................................................................................... 7 PUBLICATIONS ARISING FROM THIS THESIS.................................... 8 ACKNOWLEDGEMENTS...................................................................... 10 ABBREVIATIONS ................................................................................. 12 CHAPTER 1: INTRODUCTION ............................................................. 17 1.1 BHLH.PAS PROTEINS ............................................................................................17 1.1.1 General background..................................................................................17 1.1.2 bHLH.PAS Class I Proteins.........................................................................18 1.2 THE ARYL HYDROCARBON RECEPTOR......................................................................19 1.2.1 Domain Structure and Ligand Activation ..............................................19 1.2.2 AhR Expression and Developmental Activity .......................................21 1.2.3 Mouse AhR Knockout Phenotype ...........................................................23 -

Non-Homologous Isofunctional Enzymes: a Systematic Analysis Of
Omelchenko et al. Biology Direct 2010, 5:31 http://www.biology-direct.com/content/5/1/31 RESEARCH Open Access Non-homologousResearch isofunctional enzymes: A systematic analysis of alternative solutions in enzyme evolution Marina V Omelchenko, Michael Y Galperin*, Yuri I Wolf and Eugene V Koonin Abstract Background: Evolutionarily unrelated proteins that catalyze the same biochemical reactions are often referred to as analogous - as opposed to homologous - enzymes. The existence of numerous alternative, non-homologous enzyme isoforms presents an interesting evolutionary problem; it also complicates genome-based reconstruction of the metabolic pathways in a variety of organisms. In 1998, a systematic search for analogous enzymes resulted in the identification of 105 Enzyme Commission (EC) numbers that included two or more proteins without detectable sequence similarity to each other, including 34 EC nodes where proteins were known (or predicted) to have distinct structural folds, indicating independent evolutionary origins. In the past 12 years, many putative non-homologous isofunctional enzymes were identified in newly sequenced genomes. In addition, efforts in structural genomics resulted in a vastly improved structural coverage of proteomes, providing for definitive assessment of (non)homologous relationships between proteins. Results: We report the results of a comprehensive search for non-homologous isofunctional enzymes (NISE) that yielded 185 EC nodes with two or more experimentally characterized - or predicted - structurally unrelated proteins. Of these NISE sets, only 74 were from the original 1998 list. Structural assignments of the NISE show over-representation of proteins with the TIM barrel fold and the nucleotide-binding Rossmann fold. From the functional perspective, the set of NISE is enriched in hydrolases, particularly carbohydrate hydrolases, and in enzymes involved in defense against oxidative stress. -

Biosynthesis in Vitro of Bacillamide Intermediate-Heterocyclic Alacysthiazole by Heterologous Expression of Nonribosomal Peptide Synthetase (NRPS) T
Journal of Biotechnology 292 (2019) 5–11 Contents lists available at ScienceDirect Journal of Biotechnology journal homepage: www.elsevier.com/locate/jbiotec Biosynthesis in vitro of bacillamide intermediate-heterocyclic AlaCysthiazole by heterologous expression of nonribosomal peptide synthetase (NRPS) T Fengli Zhang, Nayila Mulati, Yukun Wang, Yingxin Li, Sanqiang Gong, Loganathan Karthik, ⁎ Wei Sun, Zhiyong Li Marine Biotechnology Laboratory, State Key Laboratory of Microbial Metabolism and School of Life Sciences & Biotechnology, Shanghai Jiao Tong University, Shanghai, China ARTICLE INFO ABSTRACT Keywords: Bacillamide C, a potential natural antialgae active compound, is produced by Bacillus atrophaeus C89 derived Bacillus atrophaeus from marine sponge Dysidea avara. A nonribosomal peptide synthetase (NRPS) cluster is hypothesized to be Bacillamides involved in the biosynthesis of bacillamide C. The NRPS with a domain string of A1-PCP1-Cy-A2-PCP2-C can be Heterologous expression divided into three functional modules. After heterologous expression and purification of module A1-PCP1 and Nonribosomal peptide synthetase (NRPS) module Cy-A2-PCP2, their catalytic activities were biochemically proven in vitro by the reaction with the apo- Thiazole PCP domain transformed to the holo-PCP domain through a phosphopantetheinyl transferase, ATP, and substrate amino acids. Five– membered heterocyclic AlaCysthiazole with molecular weight of 172.0389 was detected. This proved the formation of the heterocyclic dipeptide AlaCysthiazole, which is considered to be a building block for the biosynthesis of bacillamide. This study provides a basis for further biosynthesis of bacillamides. 1. Introduction et al., 2017). Even though the biosynthesis of bacillamide C was opti- mized, the yield was very low (Jin et al., 2011; Yu et al., 2015). -

The Microbiota-Produced N-Formyl Peptide Fmlf Promotes Obesity-Induced Glucose
Page 1 of 230 Diabetes Title: The microbiota-produced N-formyl peptide fMLF promotes obesity-induced glucose intolerance Joshua Wollam1, Matthew Riopel1, Yong-Jiang Xu1,2, Andrew M. F. Johnson1, Jachelle M. Ofrecio1, Wei Ying1, Dalila El Ouarrat1, Luisa S. Chan3, Andrew W. Han3, Nadir A. Mahmood3, Caitlin N. Ryan3, Yun Sok Lee1, Jeramie D. Watrous1,2, Mahendra D. Chordia4, Dongfeng Pan4, Mohit Jain1,2, Jerrold M. Olefsky1 * Affiliations: 1 Division of Endocrinology & Metabolism, Department of Medicine, University of California, San Diego, La Jolla, California, USA. 2 Department of Pharmacology, University of California, San Diego, La Jolla, California, USA. 3 Second Genome, Inc., South San Francisco, California, USA. 4 Department of Radiology and Medical Imaging, University of Virginia, Charlottesville, VA, USA. * Correspondence to: 858-534-2230, [email protected] Word Count: 4749 Figures: 6 Supplemental Figures: 11 Supplemental Tables: 5 1 Diabetes Publish Ahead of Print, published online April 22, 2019 Diabetes Page 2 of 230 ABSTRACT The composition of the gastrointestinal (GI) microbiota and associated metabolites changes dramatically with diet and the development of obesity. Although many correlations have been described, specific mechanistic links between these changes and glucose homeostasis remain to be defined. Here we show that blood and intestinal levels of the microbiota-produced N-formyl peptide, formyl-methionyl-leucyl-phenylalanine (fMLF), are elevated in high fat diet (HFD)- induced obese mice. Genetic or pharmacological inhibition of the N-formyl peptide receptor Fpr1 leads to increased insulin levels and improved glucose tolerance, dependent upon glucagon- like peptide-1 (GLP-1). Obese Fpr1-knockout (Fpr1-KO) mice also display an altered microbiome, exemplifying the dynamic relationship between host metabolism and microbiota. -

Whole Egg Consumption Increases Gene Expression Within the Glutathione Pathway in the Liver of Zucker Diabetic Fatty Rats
Food Science and Human Nutrition Publications Food Science and Human Nutrition 11-3-2020 Whole egg consumption increases gene expression within the glutathione pathway in the liver of Zucker Diabetic Fatty rats Joe L. Webb Iowa State University Amanda E. Bries Iowa State University, [email protected] Brooke Vogel Iowa State University Claudia Carrillo Iowa State University, [email protected] Lily Harvison Iowa State University, [email protected] See next page for additional authors Follow this and additional works at: https://lib.dr.iastate.edu/fshn_hs_pubs Part of the Dietetics and Clinical Nutrition Commons, Endocrinology, Diabetes, and Metabolism Commons, Exercise Science Commons, Food Chemistry Commons, Human and Clinical Nutrition Commons, and the Molecular, Genetic, and Biochemical Nutrition Commons The complete bibliographic information for this item can be found at https://lib.dr.iastate.edu/ fshn_hs_pubs/38. For information on how to cite this item, please visit http://lib.dr.iastate.edu/ howtocite.html. This Article is brought to you for free and open access by the Food Science and Human Nutrition at Iowa State University Digital Repository. It has been accepted for inclusion in Food Science and Human Nutrition Publications by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Whole egg consumption increases gene expression within the glutathione pathway in the liver of Zucker Diabetic Fatty rats Abstract Nutrigenomic evidence supports the idea that Type 2 Diabetes Mellitus (T2DM) arises due to the interactions between the transcriptome, individual genetic profiles, lifestyle, and diet. Since eggs are a nutrient dense food containing bioactive ingredients that modify gene expression, our goal was to examine the role of whole egg consumption on the transcriptome during T2DM. -

Shared Sulfur Mobilization Routes for Trna Thiolation and Molybdenum Cofactor Biosynthesis in Prokaryotes and Eukaryotes
biomolecules Review Shared Sulfur Mobilization Routes for tRNA Thiolation and Molybdenum Cofactor Biosynthesis in Prokaryotes and Eukaryotes Silke Leimkühler *, Martin Bühning and Lena Beilschmidt Department of Molecular Enzymology, Institute of Biochemistry and Biology, University of Potsdam, 14476 Potsdam, Germany; [email protected] (M.B.); [email protected] (L.B.) * Correspondence: [email protected]; Tel.: +49-331-977-5603 Academic Editor: Valérie de Crécy-Lagard Received: 8 December 2016; Accepted: 9 January 2017; Published: 14 January 2017 Abstract: Modifications of transfer RNA (tRNA) have been shown to play critical roles in the biogenesis, metabolism, structural stability and function of RNA molecules, and the specific modifications of nucleobases with sulfur atoms in tRNA are present in pro- and eukaryotes. Here, especially the thiomodifications xm5s2U at the wobble position 34 in tRNAs for Lys, Gln and Glu, were suggested to have an important role during the translation process by ensuring accurate deciphering of the genetic code and by stabilization of the tRNA structure. The trafficking and delivery of sulfur nucleosides is a complex process carried out by sulfur relay systems involving numerous proteins, which not only deliver sulfur to the specific tRNAs but also to other sulfur-containing molecules including iron–sulfur clusters, thiamin, biotin, lipoic acid and molybdopterin (MPT). Among the biosynthesis of these sulfur-containing molecules, the biosynthesis of the molybdenum cofactor (Moco) and the synthesis of thio-modified tRNAs in particular show a surprising link by sharing protein components for sulfur mobilization in pro- and eukaryotes. Keywords: tRNA; molybdenum cofactor; persulfide; thiocarboxylate; thionucleosides; sulfurtransferase; L-cysteine desulfurase 1. -

Harmonization Project Document No. 4
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the World Health Organization, the International Labour Organization, or the United Nations Environment Programme. Harmonization Project Document No. 4 PART 1: IPCS FRAMEWORK FOR ANALYSING THE RELEVANCE OF A CANCER MODE OF ACTION FOR HUMANS AND CASE-STUDIES PART 2: IPCS FRAMEWORK FOR ANALYSING THE RELEVANCE OF A NON-CANCER MODE OF ACTION FOR HUMANS This project was conducted within the IPCS project on the Harmonization of Approaches to the Assessment of Risk from Exposure to Chemicals. Published under the joint sponsorship of the World Health Organization, the International Labour Organization, and the United Nations Environment Programme, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals. The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organization (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals. The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and Agriculture Organization of the United Nations, WHO, the United Nations Industrial Development Organization, the United Nations Institute for Training and Research, and the Organisation for Economic Co-operation and Development (Participating Organizations), following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase coordination in the field of chemical safety. -

Human Induced Pluripotent Stem Cell–Derived Podocytes Mature Into Vascularized Glomeruli Upon Experimental Transplantation
BASIC RESEARCH www.jasn.org Human Induced Pluripotent Stem Cell–Derived Podocytes Mature into Vascularized Glomeruli upon Experimental Transplantation † Sazia Sharmin,* Atsuhiro Taguchi,* Yusuke Kaku,* Yasuhiro Yoshimura,* Tomoko Ohmori,* ‡ † ‡ Tetsushi Sakuma, Masashi Mukoyama, Takashi Yamamoto, Hidetake Kurihara,§ and | Ryuichi Nishinakamura* *Department of Kidney Development, Institute of Molecular Embryology and Genetics, and †Department of Nephrology, Faculty of Life Sciences, Kumamoto University, Kumamoto, Japan; ‡Department of Mathematical and Life Sciences, Graduate School of Science, Hiroshima University, Hiroshima, Japan; §Division of Anatomy, Juntendo University School of Medicine, Tokyo, Japan; and |Japan Science and Technology Agency, CREST, Kumamoto, Japan ABSTRACT Glomerular podocytes express proteins, such as nephrin, that constitute the slit diaphragm, thereby contributing to the filtration process in the kidney. Glomerular development has been analyzed mainly in mice, whereas analysis of human kidney development has been minimal because of limited access to embryonic kidneys. We previously reported the induction of three-dimensional primordial glomeruli from human induced pluripotent stem (iPS) cells. Here, using transcription activator–like effector nuclease-mediated homologous recombination, we generated human iPS cell lines that express green fluorescent protein (GFP) in the NPHS1 locus, which encodes nephrin, and we show that GFP expression facilitated accurate visualization of nephrin-positive podocyte formation in -
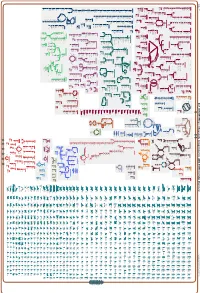
Generate Metabolic Map Poster
Authors: Pallavi Subhraveti Ron Caspi Peter Midford Peter D Karp An online version of this diagram is available at BioCyc.org. Biosynthetic pathways are positioned in the left of the cytoplasm, degradative pathways on the right, and reactions not assigned to any pathway are in the far right of the cytoplasm. Transporters and membrane proteins are shown on the membrane. Ingrid Keseler Periplasmic (where appropriate) and extracellular reactions and proteins may also be shown. Pathways are colored according to their cellular function. Gcf_003855395Cyc: Shewanella livingstonensis LMG 19866 Cellular Overview Connections between pathways are omitted for legibility. -

Structural Enzymology of Sulfide Oxidation by Persulfide Dioxygenase and Rhodanese
Structural Enzymology of Sulfide Oxidation by Persulfide Dioxygenase and Rhodanese by Nicole A. Motl A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Biological Chemistry) in the University of Michigan 2017 Doctoral Committee Professor Ruma Banerjee, Chair Assistant Professor Uhn-Soo Cho Professor Nicolai Lehnert Professor Stephen W. Ragsdale Professor Janet L. Smith Nicole A. Motl [email protected] ORCID iD: 0000-0001-6009-2988 © Nicole A. Motl 2017 ACKNOWLEDGEMENTS I would like to take this opportunity to acknowledge the many people who have provided me with guidance and support during my doctoral studies. First I would like to express my appreciation and gratitude to my advisor Dr. Ruma Banerjee for the mentorship, guidance, support and encouragement she has provided. I would like to thank my committee members Dr. Uhn-Soo Cho, Dr. Nicolai Lehnert, Dr. Stephen Ragsdale and Dr. Janet Smith for their advice, assistance and support. I would like to thank Dr. Janet Smith and members of Dr. Smith’s lab, especially Meredith Skiba, for sharing their expertise in crystallography. I would like to thank Dr. Omer Kabil for his help, suggestions and discussions in various aspects of my study. I would also like to thank members of Dr. Banerjee’s lab for their suggestions and discussions. Additionally, I would like to thank my friends and family for their support. ii TABLE OF CONTENTS ACKNOWLEDGEMENTS ii LIST OF TABLES viii LIST OF FIGURES ix ABBREVIATIONS xi ABSTRACT xii CHAPTER I. Introduction: -

Functional Investigation of Bacillus Subtilis Yrkf's Involvement in Sulfur
Peptidomics 2015; 2: 45–51 Original Study Open Access Hannah L. Martin, Katherine A. Black, Patricia C. Dos Santos* Functional investigation of Bacillus subtilis YrkF’s involvement in sulfur transfer reactions DOI 10.1515/ped-2015-0008 enzymes involved in the synthesis of the pterin organic Received August 6, 2015; accepted December 5, 2015 moiety, the insertion of sulfur and Mo to form Moco, Abstract: Sulfur incorporation into the molybdenum and the activation of their respective enzymatic partners cofactor (Moco) in the Gram-negative bacterium [4]. While components in its biosynthetic pathway vary Escherichia coli involves six enzymes. The initial between species, the structure and functionality of the reaction includes the cysteine desulfurase IscS, the active cofactor is widely conserved [5]. sulfurtransferase TusA, and the rhodanese domain- The synthesis of Moco in the Gram-negative containing protein YnjE. The Gram-positive bacterium bacterium Escherichia coli is well understood [4]. The Bacillus subtilis contains no direct homologs for IscS, organic scaffolding is synthesized from guanosine but rather four distinct cysteine desulfurases (YrvO, NifS, triphosphate (GTP) via the intermediates cyclic NifZ, SufS) and YrkF, a two-domain rhodanese protein pyranopterin monophosphate (cPMP) and molybdopterin with an N-terminal domain similar to TusA. Bioinformatic (MPT) [6], which is subsequently adenylated prior to analysis was used to identify potential enzymes involved coordinating Mo [7,8]. The conversion of cPMP to MPT in the B. subtilis Moco thiolation pathway and in vitro requires the insertion of two sulfur atoms [9], constituting reactions demonstrated that YrkF can accept sulfur from the thiolation branch of Moco biosynthesis (Figure 1). -

Auxiliary Table 2 Proteomics.Xlsx
Gene names log2(fold-Change) -Log(p-value) Significant Protein IDs Majority protein IDs Protein names gatZ -3.515 2.827 + P0C8J8;P0C8K0 P0C8J8 D-tagatose-1,6-bisphosphate aldolase subunit GatZ dps -3.362 4.596 + P0ABT2 P0ABT2 DNA protection during starvation protein sstT -3.219 3.423 + P0AGE4 P0AGE4 Ser/Thr transporter SstT iraP -3.074 3.623 + P0AAN9 P0AAN9 Anti-adapter protein IraP ilvC -3.054 3.692 + P05793 P05793 Ketol-acid reductoisomerase rfbB -3.006 4.290 + P37759 P37759 dTDP-glucose 4,6-dehydratase 1 gdhA -2.847 5.732 + P00370 P00370 NADP-specific glutamate dehydrogenase rstA -2.796 3.542 + P52108 P52108 Transcriptional regulatory protein RstA ompT -2.790 4.251 + P09169 P09169 Protease 7 uspF -2.719 4.817 + P37903 P37903 Universal stress protein F crl -2.649 4.248 + P24251 P24251 σ factor-binding protein Crl yncE -2.514 1.994 + P76116 P76116 Uncharacterized protein YncE yiiS -2.507 4.223 + P32162 P32162 UPF0381 protein YiiS yagU -2.494 2.430 + P0AAA1 P0AAA1 Inner membrane protein YagU stpA -2.488 3.540 + P0ACG1 P0ACG1 DNA-binding protein StpA ydgJ -2.476 3.366 + P77376 P77376 Uncharacterized oxidoreductase YdgJ kgtP -2.462 2.665 + P0AEX3 P0AEX3 α-ketoglutarate permease sodB -2.436 3.704 + P0AGD3 P0AGD3 Superoxide dismutase [Fe] purT -2.386 2.851 + P33221 P33221 Phosphoribosylglycinamide formyltransferase 2 trpA -2.371 3.261 + P0A877 P0A877 Trp synthase α chain acnB -2.319 2.707 + P36683 P36683 Aconitate hydratase B iadA -2.316 4.127 + P39377 P39377 Isoaspartyl dipeptidase modF -2.244 2.107 + P31060 P31060 Putative molybdenum transport