Chapter 21 Nuclear Chemistry: the Study of Nuclear Reactions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
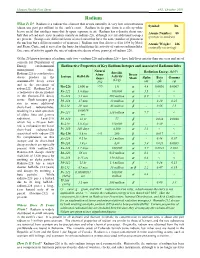
Radium What Is It? Radium Is a Radioactive Element That Occurs Naturally in Very Low Concentrations Symbol: Ra (About One Part Per Trillion) in the Earth’S Crust
Human Health Fact Sheet ANL, October 2001 Radium What Is It? Radium is a radioactive element that occurs naturally in very low concentrations Symbol: Ra (about one part per trillion) in the earth’s crust. Radium in its pure form is a silvery-white heavy metal that oxidizes immediately upon exposure to air. Radium has a density about one- Atomic Number: 88 half that of lead and exists in nature mainly as radium-226, although several additional isotopes (protons in nucleus) are present. (Isotopes are different forms of an element that have the same number of protons in the nucleus but a different number of neutrons.) Radium was first discovered in 1898 by Marie Atomic Weight: 226 and Pierre Curie, and it served as the basis for identifying the activity of various radionuclides. (naturally occurring) One curie of activity equals the rate of radioactive decay of one gram (g) of radium-226. Of the 25 known isotopes of radium, only two – radium-226 and radium-228 – have half-lives greater than one year and are of concern for Department of Energy environmental Radioactive Properties of Key Radium Isotopes and Associated Radionuclides management sites. Natural Specific Radiation Energy (MeV) Radium-226 is a radioactive Abun- Decay Isotope Half-Life Activity decay product in the dance Mode Alpha Beta Gamma (Ci/g) uranium-238 decay series (%) (α) (β) (γ) and is the precursor of Ra-226 1,600 yr >99 1.0 α 4.8 0.0036 0.0067 radon-222. Radium-228 is a radioactive decay product Rn-222 3.8 days 160,000 α 5.5 < < in the thorium-232 decay Po-218 3.1 min 290 million α 6.0 < < series. -

Nuclear Transmutation Strategies for Management of Long-Lived Fission
PRAMANA c Indian Academy of Sciences Vol. 85, No. 3 — journal of September 2015 physics pp. 517–523 Nuclear transmutation strategies for management of long-lived fission products S KAILAS1,2,∗, M HEMALATHA2 and A SAXENA1 1Nuclear Physics Division, Bhabha Atomic Research Centre, Mumbai 400 085, India 2UM–DAE Centre for Excellence in Basic Sciences, Mumbai 400 098, India ∗Corresponding author. E-mail: [email protected] DOI: 10.1007/s12043-015-1063-z; ePublication: 27 August 2015 Abstract. Management of long-lived nuclear waste produced in a reactor is essential for long- term sustenance of nuclear energy programme. A number of strategies are being explored for the effective transmutation of long-lived nuclear waste in general, and long-lived fission products (LLFP), in particular. Some of the options available for the transmutation of LLFP are discussed. Keywords. Nuclear transmutation; long-lived fission products; (n, γ ) cross-section; EMPIRE. PACS Nos 28.41.Kw; 25.40.Fq; 24.60.Dr 1. Introduction It is recognized that for long-term energy security, nuclear energy is an inevitable option [1]. For a sustainable nuclear energy programme, the management of long-lived nuclear waste is very critical. Radioactive nuclei like Pu, minor actinides like Np, Am and Cm and long-lived fission products like 79Se, 93Zr, 99Tc, 107Pd, 126Sn, 129I and 135Cs constitute the main waste burden from a power reactor. In this paper, we shall discuss the management strategies for nuclear waste in general, and long-lived fission products, in particular. 2. Management of nuclear waste The radioactive nuclei which are produced in a power reactor and which remain in the spent fuel of the reactor form a major portion of nuclear waste. -

Plasma–Material Interactions in Current Tokamaks and Their Implications for Next Step Fusion Reactors
Plasma–material interactions in current tokamaks and their implications for next step fusion reactors G. Federicia ITER Garching Joint Work Site, Garching, Germany C.H. Skinnerb Princeton Plasma Physics Laboratory, Princeton University, Princeton, New Jersey, USA J.N. Brooksc Argonne National Laboratory, Argonne, Illinois, USA J.P. Coad JET Joint Undertaking, Abingdon, United Kingdom C. Grisolia Tore Supra, CEA Cadarache, St.-Paul-lez-Durance, France A.A. Haaszd University of Toronto, Institute for Aerospace Studies, Toronto, Ontario, Canada A. Hassaneine Argonne National Laboratory, Argonne, Illinois, USA V. Philipps Institut f¨ur Plasmaphysik, Forschungzentrum J¨ulich, J¨ulich, Germany C.S. Pitcherf MIT Plasma Science and Fusion Center, Cambridge, Massachusetts, USA J. Roth Max-Planck-Institut f¨ur Plasmaphysik, Garching, Germany W.R. Wamplerg Sandia National Laboratories, Albuquerque, New Mexico, USA D.G. Whyteh University of California, San Diego, La Jolla, California, USA Nuclear Fusion, Vol. 41, No. 12R c 2001, IAEA, Vienna 1967 Contents 1. Introduction/background ........................................................................1968 1.1. Introduction....................................................................................1968 1.2. Plasma edge parameters and plasma–material interactions . 1969 1.3. History of plasma facing materials . 1973 2. Plasma edge and plasma–material interaction issues in next step tokamaks . 1977 2.1. Introduction....................................................................................1977 2.2. Progress towards a next step fusion device. .1977 2.3. Most prominent plasma–material interaction issues for a next step fusion device . 1980 2.4. Selection criteria for plasma facing materials. .1995 2.5. Overview of design features of plasma facing components for next step tokamaks. .1998 3. Review of physical processes and underlying theory ..........................................2002 3.1. Introduction....................................................................................2002 3.2. -

Royal Society of Chemistry Input to the Ad Hoc Nuclear
ROYAL SOCIETY OF CHEMISTRY INPUT TO THE AD HOC NUCLEAR RESEARCH AND DEVELOPMENT ADVISORY BOARD The Royal Society of Chemistry (RSC) was pleased to hear of the instigation of the Ad Hoc Nuclear Research and Development Advisory Board (the Board) following the findings of the House of Lords Science and Technology Committee Inquiry ‘Nuclear Research and Development Capabilities’.1,2 The RSC is the largest organisation in Europe for advancing the chemical sciences. Supported by a network of 47,000 members worldwide and an internationally acclaimed publishing business, its activities span education and training, conferences and science policy, and the promotion of the chemical sciences to the public. This document represents the views of the RSC. The RSC has a duty under its Royal Charter "to serve the public interest" by acting in an independent advisory capacity, and it is in this spirit that this submission is made. To provide input to the Board the RSC has performed a wide consultation with the chemical science community, including members of both our Radiochemistry and Energy Sector Interest Groups and also our Environment Sustainability and Energy Division. September 2012 The Role of Chemistry in a Civil Nuclear Strategy 1 Introduction Chemistry and chemical knowledge is essential in nuclear power generation and nuclear waste management. It is essential that a UK civil nuclear strategy recognises the crucial role that chemistry plays, both in research and innovation and in the development of a strong skills pipeline. As the RSC previously articulated in our response to the House of Lords Inquiry, 3 nuclear power is an important component of our current energy mix. -

The Use of Cosmic-Rays in Detecting Illicit Nuclear Materials
The use of Cosmic-Rays in Detecting Illicit Nuclear Materials Timothy Benjamin Blackwell Department of Physics and Astronomy University of Sheffield This dissertation is submitted for the degree of Doctor of Philosophy 05/05/2015 Declaration I hereby declare that except where specific reference is made to the work of others, the contents of this dissertation are original and have not been submitted in whole or in part for consideration for any other degree or qualification in this, or any other Univer- sity. This dissertation is the result of my own work and includes nothing which is the outcome of work done in collaboration, except where specifically indicated in the text. Timothy Benjamin Blackwell 05/05/2015 Acknowledgements This thesis could not have been completed without the tremendous support of many people. Firstly I would like to express special appreciation and thanks to my academic supervisor, Dr Vitaly A. Kudryavtsev for his expertise, understanding and encourage- ment. I have enjoyed our many discussions concerning my research topic throughout the PhD journey. I would also like to thank my viva examiners, Professor Lee Thomp- son and Dr Chris Steer, for the time you have taken out of your schedules, so that I may take this next step in my career. Thanks must also be given to Professor Francis Liven, Professor Neil Hyatt and the rest of the Nuclear FiRST DTC team, for the initial oppor- tunity to pursue postgraduate research. Appreciation is also given to the University of Sheffield, the HEP group and EPRSC for providing me with the facilities and funding, during this work. -

Chapter 3 the Fundamentals of Nuclear Physics Outline Natural
Outline Chapter 3 The Fundamentals of Nuclear • Terms: activity, half life, average life • Nuclear disintegration schemes Physics • Parent-daughter relationships Radiation Dosimetry I • Activation of isotopes Text: H.E Johns and J.R. Cunningham, The physics of radiology, 4th ed. http://www.utoledo.edu/med/depts/radther Natural radioactivity Activity • Activity – number of disintegrations per unit time; • Particles inside a nucleus are in constant motion; directly proportional to the number of atoms can escape if acquire enough energy present • Most lighter atoms with Z<82 (lead) have at least N Average one stable isotope t / ta A N N0e lifetime • All atoms with Z > 82 are radioactive and t disintegrate until a stable isotope is formed ta= 1.44 th • Artificial radioactivity: nucleus can be made A N e0.693t / th A 2t / th unstable upon bombardment with neutrons, high 0 0 Half-life energy protons, etc. • Units: Bq = 1/s, Ci=3.7x 1010 Bq Activity Activity Emitted radiation 1 Example 1 Example 1A • A prostate implant has a half-life of 17 days. • A prostate implant has a half-life of 17 days. If the What percent of the dose is delivered in the first initial dose rate is 10cGy/h, what is the total dose day? N N delivered? t /th t 2 or e Dtotal D0tavg N0 N0 A. 0.5 A. 9 0.693t 0.693t B. 2 t /th 1/17 t 2 2 0.96 B. 29 D D e th dt D h e th C. 4 total 0 0 0.693 0.693t /th 0.6931/17 C. -

Electron Capture in Stars
Electron capture in stars K Langanke1;2, G Mart´ınez-Pinedo1;2;3 and R.G.T. Zegers4;5;6 1GSI Helmholtzzentrum f¨urSchwerionenforschung, D-64291 Darmstadt, Germany 2Institut f¨urKernphysik (Theoriezentrum), Department of Physics, Technische Universit¨atDarmstadt, D-64298 Darmstadt, Germany 3Helmholtz Forschungsakademie Hessen f¨urFAIR, GSI Helmholtzzentrum f¨ur Schwerionenforschung, D-64291 Darmstadt, Germany 4 National Superconducting Cyclotron Laboratory, Michigan State University, East Lansing, Michigan 48824, USA 5 Joint Institute for Nuclear Astrophysics: Center for the Evolution of the Elements, Michigan State University, East Lansing, Michigan 48824, USA 6 Department of Physics and Astronomy, Michigan State University, East Lansing, Michigan 48824, USA E-mail: [email protected], [email protected], [email protected] Abstract. Electron captures on nuclei play an essential role for the dynamics of several astrophysical objects, including core-collapse and thermonuclear supernovae, the crust of accreting neutron stars in binary systems and the final core evolution of intermediate mass stars. In these astrophysical objects, the capture occurs at finite temperatures and at densities at which the electrons form a degenerate relativistic electron gas. The capture rates can be derived in perturbation theory where allowed nuclear transitions (Gamow-Teller transitions) dominate, except at the higher temperatures achieved in core-collapse supernovae where also forbidden transitions contribute significantly to the rates. There has been decisive progress in recent years in measuring Gamow-Teller (GT) strength distributions using novel experimental techniques based on charge-exchange reactions. These measurements provide not only data for the GT distributions of ground states for many relevant nuclei, but also serve as valuable constraints for nuclear models which are needed to derive the capture rates for the arXiv:2009.01750v1 [nucl-th] 3 Sep 2020 many nuclei, for which no data exist yet. -

Two-Proton Radioactivity 2
Two-proton radioactivity Bertram Blank ‡ and Marek P loszajczak † ‡ Centre d’Etudes Nucl´eaires de Bordeaux-Gradignan - Universit´eBordeaux I - CNRS/IN2P3, Chemin du Solarium, B.P. 120, 33175 Gradignan Cedex, France † Grand Acc´el´erateur National d’Ions Lourds (GANIL), CEA/DSM-CNRS/IN2P3, BP 55027, 14076 Caen Cedex 05, France Abstract. In the first part of this review, experimental results which lead to the discovery of two-proton radioactivity are examined. Beyond two-proton emission from nuclear ground states, we also discuss experimental studies of two-proton emission from excited states populated either by nuclear β decay or by inelastic reactions. In the second part, we review the modern theory of two-proton radioactivity. An outlook to future experimental studies and theoretical developments will conclude this review. PACS numbers: 23.50.+z, 21.10.Tg, 21.60.-n, 24.10.-i Submitted to: Rep. Prog. Phys. Version: 17 December 2013 arXiv:0709.3797v2 [nucl-ex] 23 Apr 2008 Two-proton radioactivity 2 1. Introduction Atomic nuclei are made of two distinct particles, the protons and the neutrons. These nucleons constitute more than 99.95% of the mass of an atom. In order to form a stable atomic nucleus, a subtle equilibrium between the number of protons and neutrons has to be respected. This condition is fulfilled for 259 different combinations of protons and neutrons. These nuclei can be found on Earth. In addition, 26 nuclei form a quasi stable configuration, i.e. they decay with a half-life comparable or longer than the age of the Earth and are therefore still present on Earth. -

2.3 Neutrino-Less Double Electron Capture - Potential Tool to Determine the Majorana Neutrino Mass by Z.Sujkowski, S Wycech
DEPARTMENT OF NUCLEAR SPECTROSCOPY AND TECHNIQUE 39 The above conservatively large systematic hypothesis. TIle quoted uncertainties will be soon uncertainty reflects the fact that we did not finish reduced as our analysis progresses. evaluating the corrections fully in the current analysis We are simultaneously recording a large set of at the time of this writing, a situation that will soon radiative decay events for the processes t e'v y change. This result is to be compared with 1he and pi-+eN v y. The former will be used to extract previous most accurate measurement of McFarlane the ratio FA/Fv of the axial and vector form factors, a et al. (Phys. Rev. D 1984): quantity of great and longstanding interest to low BR = (1.026 ± 0.039)'1 I 0 energy effective QCD theory. Both processes are as well as with the Standard Model (SM) furthermore very sensitive to non- (V-A) admixtures in prediction (Particle Data Group - PDG 2000): the electroweak lagLangian, and thus can reveal BR = (I 038 - 1.041 )*1 0-s (90%C.L.) information on physics beyond the SM. We are currently analyzing these data and expect results soon. (1.005 - 1.008)* 1W') - excl. rad. corr. Tale 1 We see that even working result strongly confirms Current P1IBETA event sxpelilnentstatistics, compared with the the validity of the radiative corrections. Another world data set. interesting comparison is with the prediction based on Decay PIBETA World data set the most accurate evaluation of the CKM matrix n >60k 1.77k element V d based on the CVC hypothesis and ihce >60 1.77_ _ _ results -

Nuclear Chemistry Card Sort
Nuclear Chemistry Card Sort Submitted by: Annette Gillespie, Ashely Gilomen, Sarah Johnson, and Stephanie Kimberlin Appalachian Regional Commission/Oak Ridge National Laboratory Summer Math-Science-Technology Institute Grade: 9-12 Lesson Duration: 45-60 minutes Materials Needed: • Media for Venn Diagram (e.g., poster board, white board, butcher paper, electronic devices) • One copy of the list of nuclear chemistry concepts/nuclear chemistry concept cards provided in this lesson plan • Envelope for storing cut-up list/cards Background Information: Students should be able to describe the basic structure of an atom and have a basic understanding of nuclear notation. Lesson Objective: Students will be able to • Describe the basic principles of nuclear chemistry. • Discern between diagrams and representations of nuclear equations and processes. • Use context clues to correctly categorize nuclear events. Instructional Process: All informative resources should be made available to the students so they can categorize the concepts (e.g., notes, text books, Internet). This lesson is best suited as an introduction activity for the beginning of the nuclear chemistry unit and/or as a review activity at the end of the nuclear chemistry unit. Students should be organized into groups of 3-4 to complete this activity. Each student group should create and label a three-circle Venn diagram on the teacher-designated media. (Refer to the examples provided in figure 1 and at the end of this lesson plan.) Each group should receive one copy of the list of nuclear chemistry concepts/nuclear chemistry concept cards, which they will cut up into individual cards. Once students have their diagrams drawn and cards cut out, they should place the nuclear chemistry concepts cards into one of three categories on the diagram: fission, fusion, or nuclear decay. -
Unit 1 – Atomic Structure and Nuclear Chemistry Introduction to the Atom
Unit 1 – Atomic Structure and Nuclear Chemistry Introduction to the atom Modern Atomic Theory All matter is composed of atoms Atoms cannot be subdivided, created, or destroyed in ordinary chemical reactions. However, these changes CAN occur in nuclear reactions! Every atom has different properties from other atoms Ex: grinding down a gold ring Modern Atomic Theory Wait, it’s “only” a theory? Why are we learning it then? •A theory is a powerful term in science Theory -A set of tested hypotheses that gives an overall explanation of some natural phenomenon. Ex: Cell theory & Evolutionary theory We can now see atoms …sort of In 1981 a STM (Scanning Tunneling Microscope) was created. - We can see them and manipulate them. The Kanji characters for "atom." This image was formed by using the tiny tip of an STM to pick up individual atoms of iron and place them on a copper (111) surface. Nanotechnology is coming Atoms can be moved and molded to make various devices such as molecular motors Structure of the Atom Accessing Prior Knowledge 1. Based on your previous science classes, draw a generic atom and label where you’d find the nucleus, protons, neutrons, & electrons. 2. For a common beryllium atom, what is the: a) # protons? b) # neutrons? c) # electrons? Structure of an Atom Electrons (in electron cloud) 1/2000th the mass of P+ & N Nucleus (protons + neutrons) Particle Charge Mass Location Purpose # Electron -1 0 Electron Behavior of cloud element Proton +1 1 Nucleus Identity of element Neutron 0 1 Nucleus Stability of nucleus Charges in an Atom The atom is generally neutral because: # of negative electrons = # of positive protons The nucleus is positively charged because: Contains positive protons (and neutrons which don’t have a charge). -

Annual Report of Institute of Nuclear Chemistry and Technology 1996
PL9702079 ISSN 1425-ZU4A IN/&~PL- 003 INSTITUTE OF NUCLEAR CHEMISTRY AND TECHNOLOGY m DO /? WMEM\ 1996 OF NUCLEI AND TECHNOLOGY 1996 EDITORIAL BOARD Wiktor Smuiek, Ph.D. Ewa Godlewska Sylwester Wojtas © Copyright by the Institute of Nuclear Chemistry and Technology, Warszawa 1997 All rights reserved CONTENTS GENERAL INFORMATION 7 MANAGEMENT OF THE INSTITUTE 9 MANAGING STAFF OF THE INSTITUTE 9 HEADS OF THE INCT DEPARTMENTS 9 PROFESSORS AND SCIENTIFIC COUNCIL 10 PROFESSORS 10 ASSOCIATE PROFESSORS 10 ASSISTANT PROFESSORS (Ph.D.) 11 SCIENTIFIC COUNCIL (1995-1999) 13 HONORARY MEMBERS OF THE INCT SCIENTIFIC COUNCIL (1995-1999) 14 RADIATION CHEMISTRY AND PHYSICS 15 ONE-ELECTRON OXIDATION OF METALLOPORPHYCENES AS STUDIED BY RADIOLYTIC METHODS D M. Guldi, J. Field, J. Grodkowski, P. Neta, E. Vogel 17 IRON-PORPHYRIN CATALYZED REDUCTION OF CO& PHOTOCHEMICAL AND RADIATION CHEMICAL STUDIES J. Grodkowski, D. Behar, P. Neta, P. Hambright 17 RATE CONSTANTS FOR REACTIONS OF ALIPHATIC CARBON-CENTERED RADICALS IN AQUEOUS SOLUTION P. Neta, J. Grodkowski, A.B. Ross 19 TETRABENZOPORPHYRINS: METAL INCORPORATION AND EXCHANGE KINETICS, LIGATIONAL EQUILIBRIA AND PULSE RADIOLYSIS STUDY S. Cromer, P. Hambright, J. Grodkowski, P. Neta 19 REDUCTION AND ALKYLATION OF RHODIUM PORPHYRINS IN ALCOHOL SOLUTIONS. RADIATION CHEMICAL AND PHOTOCHEMICAL STUDIES J. Grodkowski, P. Neta, Y. Abdallach, P. Hambright 20 •OH RADICAL INDUCED OXIDATION OF S-METHYLCYSTEINE AND ITS DERIVATIVES FRAGMENTATION OF a-AMINOALKYL RADICALS D. Pogocki, K. Bobrowski, K.-D. Asmus 21 Trp ' -* iyrO RADICAL TRANSFOMATION IN HEN-EGG WHITE LYSOZYME K. Bobrowski, J. Holcman, J. Poznafiski, K.L. Wierzchowski 24 SENSITIZED PHOTOOXIDATION OF METHIONINE-CONTAINING PEPTIDES IN AQUEOUS SOLUTION K.