Introduction to Nuclear Physics and Nuclear Decay
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

B2.IV Nuclear and Particle Physics
B2.IV Nuclear and Particle Physics A.J. Barr February 13, 2014 ii Contents 1 Introduction 1 2 Nuclear 3 2.1 Structure of matter and energy scales . 3 2.2 Binding Energy . 4 2.2.1 Semi-empirical mass formula . 4 2.3 Decays and reactions . 8 2.3.1 Alpha Decays . 10 2.3.2 Beta decays . 13 2.4 Nuclear Scattering . 18 2.4.1 Cross sections . 18 2.4.2 Resonances and the Breit-Wigner formula . 19 2.4.3 Nuclear scattering and form factors . 22 2.5 Key points . 24 Appendices 25 2.A Natural units . 25 2.B Tools . 26 2.B.1 Decays and the Fermi Golden Rule . 26 2.B.2 Density of states . 26 2.B.3 Fermi G.R. example . 27 2.B.4 Lifetimes and decays . 27 2.B.5 The flux factor . 28 2.B.6 Luminosity . 28 2.C Shell Model § ............................. 29 2.D Gamma decays § ............................ 29 3 Hadrons 33 3.1 Introduction . 33 3.1.1 Pions . 33 3.1.2 Baryon number conservation . 34 3.1.3 Delta baryons . 35 3.2 Linear Accelerators . 36 iii CONTENTS CONTENTS 3.3 Symmetries . 36 3.3.1 Baryons . 37 3.3.2 Mesons . 37 3.3.3 Quark flow diagrams . 38 3.3.4 Strangeness . 39 3.3.5 Pseudoscalar octet . 40 3.3.6 Baryon octet . 40 3.4 Colour . 41 3.5 Heavier quarks . 43 3.6 Charmonium . 45 3.7 Hadron decays . 47 Appendices 48 3.A Isospin § ................................ 49 3.B Discovery of the Omega § ...................... -
Pair Energy of Proton and Neutron in Atomic Nuclei
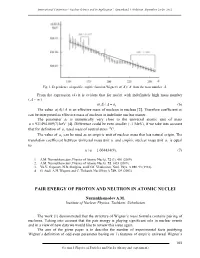
International Conference “Nuclear Science and its Application”, Samarkand, Uzbekistan, September 25-28, 2012 Fig. 1. Dependence of specific empiric function Wigner's a()/ A A from the mass number A. From the expression (4) it is evident that for nuclei with indefinitely high mass number ( A ~ ) a()/. A A a1 (6) The value a()/ A A is an effective mass of nucleon in nucleus [2]. Therefore coefficient a1 can be interpreted as effective mass of nucleon in indefinite nuclear matter. The parameter a1 is numerically very close to the universal atomic unit of mass u 931494.009(7) keV [4]. Difference could be even smaller (~1 MeV), if we take into account that for definition of u, used mass of neutral atom 12C. The value of a1 can be used as an empiric unit of nuclear mass that has natural origin. The translation coefficient between universal mass unit u and empiric nuclear mass unit a1 is equal to: u/ a1 1.004434(9). (7) 1. A.M. Nurmukhamedov, Physics of Atomic Nuclei, 72 (3), 401 (2009). 2. A.M. Nurmukhamedov, Physics of Atomic Nuclei, 72, 1435 (2009). 3. Yu.V. Gaponov, N.B. Shulgina, and D.M. Vladimirov, Nucl. Phys. A 391, 93 (1982). 4. G. Audi, A.H. Wapstra and C. Thibault, Nucl.Phys A 729, 129 (2003). PAIR ENERGY OF PROTON AND NEUTRON IN ATOMIC NUCLEI Nurmukhamedov A.M. Institute of Nuclear Physics, Tashkent, Uzbekistan The work [1] demonstrated that the structure of Wigner’s mass formula contains pairing of nucleons. Taking into account that the pair energy is playing significant role in nuclear events and in a view of new data we would like to review this issue again. -

Chapter 3 the Fundamentals of Nuclear Physics Outline Natural
Outline Chapter 3 The Fundamentals of Nuclear • Terms: activity, half life, average life • Nuclear disintegration schemes Physics • Parent-daughter relationships Radiation Dosimetry I • Activation of isotopes Text: H.E Johns and J.R. Cunningham, The physics of radiology, 4th ed. http://www.utoledo.edu/med/depts/radther Natural radioactivity Activity • Activity – number of disintegrations per unit time; • Particles inside a nucleus are in constant motion; directly proportional to the number of atoms can escape if acquire enough energy present • Most lighter atoms with Z<82 (lead) have at least N Average one stable isotope t / ta A N N0e lifetime • All atoms with Z > 82 are radioactive and t disintegrate until a stable isotope is formed ta= 1.44 th • Artificial radioactivity: nucleus can be made A N e0.693t / th A 2t / th unstable upon bombardment with neutrons, high 0 0 Half-life energy protons, etc. • Units: Bq = 1/s, Ci=3.7x 1010 Bq Activity Activity Emitted radiation 1 Example 1 Example 1A • A prostate implant has a half-life of 17 days. • A prostate implant has a half-life of 17 days. If the What percent of the dose is delivered in the first initial dose rate is 10cGy/h, what is the total dose day? N N delivered? t /th t 2 or e Dtotal D0tavg N0 N0 A. 0.5 A. 9 0.693t 0.693t B. 2 t /th 1/17 t 2 2 0.96 B. 29 D D e th dt D h e th C. 4 total 0 0 0.693 0.693t /th 0.6931/17 C. -

A Measurement of the 2 Neutrino Double Beta Decay Rate of 130Te in the CUORICINO Experiment by Laura Katherine Kogler
A measurement of the 2 neutrino double beta decay rate of 130Te in the CUORICINO experiment by Laura Katherine Kogler A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Physics in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Stuart J. Freedman, Chair Professor Yury G. Kolomensky Professor Eric B. Norman Fall 2011 A measurement of the 2 neutrino double beta decay rate of 130Te in the CUORICINO experiment Copyright 2011 by Laura Katherine Kogler 1 Abstract A measurement of the 2 neutrino double beta decay rate of 130Te in the CUORICINO experiment by Laura Katherine Kogler Doctor of Philosophy in Physics University of California, Berkeley Professor Stuart J. Freedman, Chair CUORICINO was a cryogenic bolometer experiment designed to search for neutrinoless double beta decay and other rare processes, including double beta decay with two neutrinos (2νββ). The experiment was located at Laboratori Nazionali del Gran Sasso and ran for a period of about 5 years, from 2003 to 2008. The detector consisted of an array of 62 TeO2 crystals arranged in a tower and operated at a temperature of ∼10 mK. Events depositing energy in the detectors, such as radioactive decays or impinging particles, produced thermal pulses in the crystals which were read out using sensitive thermistors. The experiment included 4 enriched crystals, 2 enriched with 130Te and 2 with 128Te, in order to aid in the measurement of the 2νββ rate. The enriched crystals contained a total of ∼350 g 130Te. The 128-enriched (130-depleted) crystals were used as background monitors, so that the shared backgrounds could be subtracted from the energy spectrum of the 130- enriched crystals. -

Compilation and Evaluation of Fission Yield Nuclear Data Iaea, Vienna, 2000 Iaea-Tecdoc-1168 Issn 1011–4289
IAEA-TECDOC-1168 Compilation and evaluation of fission yield nuclear data Final report of a co-ordinated research project 1991–1996 December 2000 The originating Section of this publication in the IAEA was: Nuclear Data Section International Atomic Energy Agency Wagramer Strasse 5 P.O. Box 100 A-1400 Vienna, Austria COMPILATION AND EVALUATION OF FISSION YIELD NUCLEAR DATA IAEA, VIENNA, 2000 IAEA-TECDOC-1168 ISSN 1011–4289 © IAEA, 2000 Printed by the IAEA in Austria December 2000 FOREWORD Fission product yields are required at several stages of the nuclear fuel cycle and are therefore included in all large international data files for reactor calculations and related applications. Such files are maintained and disseminated by the Nuclear Data Section of the IAEA as a member of an international data centres network. Users of these data are from the fields of reactor design and operation, waste management and nuclear materials safeguards, all of which are essential parts of the IAEA programme. In the 1980s, the number of measured fission yields increased so drastically that the manpower available for evaluating them to meet specific user needs was insufficient. To cope with this task, it was concluded in several meetings on fission product nuclear data, some of them convened by the IAEA, that international co-operation was required, and an IAEA co-ordinated research project (CRP) was recommended. This recommendation was endorsed by the International Nuclear Data Committee, an advisory body for the nuclear data programme of the IAEA. As a consequence, the CRP on the Compilation and Evaluation of Fission Yield Nuclear Data was initiated in 1991, after its scope, objectives and tasks had been defined by a preparatory meeting. -

Electron Capture in Stars
Electron capture in stars K Langanke1;2, G Mart´ınez-Pinedo1;2;3 and R.G.T. Zegers4;5;6 1GSI Helmholtzzentrum f¨urSchwerionenforschung, D-64291 Darmstadt, Germany 2Institut f¨urKernphysik (Theoriezentrum), Department of Physics, Technische Universit¨atDarmstadt, D-64298 Darmstadt, Germany 3Helmholtz Forschungsakademie Hessen f¨urFAIR, GSI Helmholtzzentrum f¨ur Schwerionenforschung, D-64291 Darmstadt, Germany 4 National Superconducting Cyclotron Laboratory, Michigan State University, East Lansing, Michigan 48824, USA 5 Joint Institute for Nuclear Astrophysics: Center for the Evolution of the Elements, Michigan State University, East Lansing, Michigan 48824, USA 6 Department of Physics and Astronomy, Michigan State University, East Lansing, Michigan 48824, USA E-mail: [email protected], [email protected], [email protected] Abstract. Electron captures on nuclei play an essential role for the dynamics of several astrophysical objects, including core-collapse and thermonuclear supernovae, the crust of accreting neutron stars in binary systems and the final core evolution of intermediate mass stars. In these astrophysical objects, the capture occurs at finite temperatures and at densities at which the electrons form a degenerate relativistic electron gas. The capture rates can be derived in perturbation theory where allowed nuclear transitions (Gamow-Teller transitions) dominate, except at the higher temperatures achieved in core-collapse supernovae where also forbidden transitions contribute significantly to the rates. There has been decisive progress in recent years in measuring Gamow-Teller (GT) strength distributions using novel experimental techniques based on charge-exchange reactions. These measurements provide not only data for the GT distributions of ground states for many relevant nuclei, but also serve as valuable constraints for nuclear models which are needed to derive the capture rates for the arXiv:2009.01750v1 [nucl-th] 3 Sep 2020 many nuclei, for which no data exist yet. -

Lecture 22 Alpha Decay 1 Introduction 2 Fission and Fusion
Nuclear and Particle Physics - Lecture 22 Alpha decay 1 Introduction We have looked at gamma decays (due to the EM force) and beta decays (due to the weak force) and now will look at alpha decays, which are due to the strong/nuclear force. In contrast to the previous decays which do not change A, alpha decays happen by emission of some of the 4 nucleons from the nucleus. Specifically, for alpha decay, an alpha particle, 2He, is ejected so generically A A−4 4 X − Y + He Z !Z 2 2 for some X and Y . Y clearly has a different number of nucleons to X. Compare how alpha and beta decays move the nuclei around in Z,N plane N β+ β- α Z Note that gamma decays cannot change Z, N or A. 2 Fission and fusion Alpha decay is in fact only one specific case of a whole range of processes which involve emission of nucleons. These range from single proton or neutron emission up to splitting the nucleus into two roughly equal parts. Particularly in the latter case, these are called fission decays. Which nuclei would we expect to fission? The binding energy per nucleon curve shows that 56 the maximum occurs around 26Fe and drops off to either side. Hence, both small A and large A nuclei are less strongly bound per nucleon than medium A. This means there will be some energy release if two small nuclei are combined into a larger one, a process called fusion which will be discussed later in the course. -

Radioactive Decay
North Berwick High School Department of Physics Higher Physics Unit 2 Particles and Waves Section 3 Fission and Fusion Section 3 Fission and Fusion Note Making Make a dictionary with the meanings of any new words. Einstein and nuclear energy 1. Write down Einstein’s famous equation along with units. 2. Explain the importance of this equation and its relevance to nuclear power. A basic model of the atom 1. Copy the components of the atom diagram and state the meanings of A and Z. 2. Copy the table on page 5 and state the difference between elements and isotopes. Radioactive decay 1. Explain what is meant by radioactive decay and copy the summary table for the three types of nuclear radiation. 2. Describe an alpha particle, including the reason for its short range and copy the panel showing Plutonium decay. 3. Describe a beta particle, including its range and copy the panel showing Tritium decay. 4. Describe a gamma ray, including its range. Fission: spontaneous decay and nuclear bombardment 1. Describe the differences between the two methods of decay and copy the equation on page 10. Nuclear fission and E = mc2 1. Explain what is meant by the terms ‘mass difference’ and ‘chain reaction’. 2. Copy the example showing the energy released during a fission reaction. 3. Briefly describe controlled fission in a nuclear reactor. Nuclear fusion: energy of the future? 1. Explain why nuclear fusion might be a preferred source of energy in the future. 2. Describe some of the difficulties associated with maintaining a controlled fusion reaction. -
![Arxiv:1901.01410V3 [Astro-Ph.HE] 1 Feb 2021 Mental Information Is Available, and One Has to Rely Strongly on Theoretical Predictions for Nuclear Properties](https://docslib.b-cdn.net/cover/8159/arxiv-1901-01410v3-astro-ph-he-1-feb-2021-mental-information-is-available-and-one-has-to-rely-strongly-on-theoretical-predictions-for-nuclear-properties-508159.webp)
Arxiv:1901.01410V3 [Astro-Ph.HE] 1 Feb 2021 Mental Information Is Available, and One Has to Rely Strongly on Theoretical Predictions for Nuclear Properties
Origin of the heaviest elements: The rapid neutron-capture process John J. Cowan∗ HLD Department of Physics and Astronomy, University of Oklahoma, 440 W. Brooks St., Norman, OK 73019, USA Christopher Snedeny Department of Astronomy, University of Texas, 2515 Speedway, Austin, TX 78712-1205, USA James E. Lawlerz Physics Department, University of Wisconsin-Madison, 1150 University Avenue, Madison, WI 53706-1390, USA Ani Aprahamianx and Michael Wiescher{ Department of Physics and Joint Institute for Nuclear Astrophysics, University of Notre Dame, 225 Nieuwland Science Hall, Notre Dame, IN 46556, USA Karlheinz Langanke∗∗ GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany and Institut f¨urKernphysik (Theoriezentrum), Fachbereich Physik, Technische Universit¨atDarmstadt, Schlossgartenstraße 2, 64298 Darmstadt, Germany Gabriel Mart´ınez-Pinedoyy GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany; Institut f¨urKernphysik (Theoriezentrum), Fachbereich Physik, Technische Universit¨atDarmstadt, Schlossgartenstraße 2, 64298 Darmstadt, Germany; and Helmholtz Forschungsakademie Hessen f¨urFAIR, GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany Friedrich-Karl Thielemannzz Department of Physics, University of Basel, Klingelbergstrasse 82, 4056 Basel, Switzerland and GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany (Dated: February 2, 2021) The production of about half of the heavy elements found in nature is assigned to a spe- cific astrophysical nucleosynthesis process: the rapid neutron capture process (r-process). Although this idea has been postulated more than six decades ago, the full understand- ing faces two types of uncertainties/open questions: (a) The nucleosynthesis path in the nuclear chart runs close to the neutron-drip line, where presently only limited experi- arXiv:1901.01410v3 [astro-ph.HE] 1 Feb 2021 mental information is available, and one has to rely strongly on theoretical predictions for nuclear properties. -

Cooperative Internal Conversion Process by Proton Exchange
Cooperative internal conversion process by proton exchange P´eter K´alm´an∗ and Tam´as Keszthelyi† Budapest University of Technology and Economics, Institute of Physics, Budafoki ´ut 8. F., H-1521 Budapest, Hungary A generalization of the recently discovered cooperative internal conversion process is investigated theoretically. In the cooperative internal conversion process by proton exchange investigated the coupling of bound-free electron and proton transitions due to the dipole term of their Coulomb interaction permits cooperation of two nuclei leading to proton exchange and an electron emission. General expression of the cross section of the process obtained in the one particle spherical nuclear shell model is presented. As a numerical example the cooperative internal conversion process by proton exchange in Al is dealt with. As a further generalization, cooperative internal conversion process by heavy charged particle exchange and as an example of it the cooperative internal con- version process by triton exchange is discussed. The process is also connected to the field of nuclear waste disposal. PACS numbers: 23.20.Nx, 25.90.+k, 28.41.Kw, Keywords: internal conversion and extranuclear effects, other topics of nuclear reactions: specific reactions, radioactive wastes, waste disposal In a recent paper [1] a new phenomenon, the coop- erative internal conversion process (CICP) is discussed which is a special type of the well known internal conver- sion process [2]. In CICP two nuclei cooperate by neutron exchange creating final nuclei of energy lower than the en- ergy of the initial nuclei. The process is initiated by the coupling of bound-free electron and neutron transitions due to the dipole term of their Coulomb interaction in the initial atom leading to the creation of a virtual free neutron which is captured through strong interaction by an other nucleus. -

Hadronic Physics II
Geant4 10.1 p01 Hadronic Physics II Geant4 Tutorial at M&C+SNA+MC2015 19 April 2015 Dennis Wright (SLAC) Outline • Low energy hadronic models • Capture, Stopping and Fission • Gamma- and lepto-nuclear models • RadioacQve decay 2 Low Energy Neutron Physics • Below 20 MeV incident energy, Geant4 provides several models for treang neutron interacQons in detail • The high precision models (NeutronHP) are data-driven and depend on a large database of cross secQons, etc. • the G4NDL database is available for download from the Geant4 web site • elasQc, inelasQc, capture and fission models all use this isotope- dependent data • There are also models to handle thermal scaering from chemically bound atoms 3 Geant4 Neutron Data Library (G4NDL) • Contains the data files for the high precision neutron models • includes both cross secQons and final states • From Geant4 9.5 onward, G4NDL is based solely on the ENDF/B-VII database • G4NDL data is now taken only from ENDF/B-VII, but sQll has G4NDL format • use G4NDL 4.0 or later • Prior to G4 9.5 G4NDL selected data from 9 different databases, each with its own format • Brond-2.1, CENDL2.2, EFF-3, ENDF/B-VI, FENDL/E2.0, JEF2.2, JENDL-FF, JENDL-3 and MENDL-2 • G4NDL also had its own (undocumented) format 4 G4NeutronHPElasQc • Handles elasQc scaering of neutrons by sampling differenQal cross secQon data • interpolates between points in the cross secQon tables as a funcon of energy • also interpolates between Legendre polynomial coefficients to get the angular distribuQon as a funcQon of energy • scaered neutron and recoil nucleus generated as final state • Note that because look-up tables are based on binned data, there will always be a small energy non-conservaon • true for inelasQc, capture and fission processes as well 5 G4NeutronHPInelasQc • Currently supports 34 inelasQc final states + n gamma (discrete and conQnuum) • n (A,Z) -> (A-1, Z-1) n p • n (A,Z) -> (A-3, Z) n n n n • n (A,Z) -> (A-4, Z-2) d t • ……. -

2.3 Neutrino-Less Double Electron Capture - Potential Tool to Determine the Majorana Neutrino Mass by Z.Sujkowski, S Wycech
DEPARTMENT OF NUCLEAR SPECTROSCOPY AND TECHNIQUE 39 The above conservatively large systematic hypothesis. TIle quoted uncertainties will be soon uncertainty reflects the fact that we did not finish reduced as our analysis progresses. evaluating the corrections fully in the current analysis We are simultaneously recording a large set of at the time of this writing, a situation that will soon radiative decay events for the processes t e'v y change. This result is to be compared with 1he and pi-+eN v y. The former will be used to extract previous most accurate measurement of McFarlane the ratio FA/Fv of the axial and vector form factors, a et al. (Phys. Rev. D 1984): quantity of great and longstanding interest to low BR = (1.026 ± 0.039)'1 I 0 energy effective QCD theory. Both processes are as well as with the Standard Model (SM) furthermore very sensitive to non- (V-A) admixtures in prediction (Particle Data Group - PDG 2000): the electroweak lagLangian, and thus can reveal BR = (I 038 - 1.041 )*1 0-s (90%C.L.) information on physics beyond the SM. We are currently analyzing these data and expect results soon. (1.005 - 1.008)* 1W') - excl. rad. corr. Tale 1 We see that even working result strongly confirms Current P1IBETA event sxpelilnentstatistics, compared with the the validity of the radiative corrections. Another world data set. interesting comparison is with the prediction based on Decay PIBETA World data set the most accurate evaluation of the CKM matrix n >60k 1.77k element V d based on the CVC hypothesis and ihce >60 1.77_ _ _ results