New Insights Into Drug Discovery Targeting Tau Protein
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Surnames and Migrations: the Barcelona Area (1451-1900)1
Surnames and Migrations: The Barcelona Area (1451-1900)1 Joan Pau Jordà Joana Maria Pujadas-Mora Anna Cabré Spain Abstract Catalan onomastics, and specifically the evolution of surnames, has been conditioned by several demographic, political and social processes that have imparted singular characteristics over the course of centuries. The combination of these factors resulted in a significant number of homonymic surnames, making it impossible to correctly identify their geographical origin based solely on linguistic criteria. As a possible solution to this, this paper proposes the use of the cluster analysis method to introduce a further criterion for their identification and classification. Historical registers of Marriage License Books from the Diocese of Barcelona are the source selected to achieve this goal. These records, which collect information on more than two million surnames, were maintained between 1451 and 1905 in a set of 291 books (Llibres d’Esposalles) kept at the archives of the Barcelona Cathedral. * * * Introduction The study of historical migrations is one of the most difficult demographic phenomena to investigate due to the absence of specific records until recent times. Given this lack, it is necessary to rely on indirect sources and methods that have already shown great potential, such as the analysis of surnames.2 However in the Catalan case – as well as in others – the evolution of onomastics, and specifically the evolution of surnames, has been conditioned by several demographic, political and social factors that have imparted singular characteristics over the course of centuries. The combination of these processes, as explained below, has made it necessary to propose the use of complementary methods to correctly identify the geographical origin of surnames and to complement existing linguistic criteria. -

Men Final Entries
Final Entries - Athletes List by event European Athletics Indoor Championships 2021, Torun/POL Tot. Number of countries Tot. Number of athletes Tot. Number of Men Tot. Number of Women 47 733 405 328 FINAL ENTRIES - Men 60m Senior Men Num. of countries: 33 Num. of athletes: 71 Member Federation Surname First Name DoB PB SB ARM Donigian Alexander 20/10/1993 6.64i 6.79i ART Keletela Dorian Celeste 06/02/1999 6.79i 6.85i AUT Fuchs Markus 14/11/1995 6.62i 6.69i BEL Kuba Di-Vita Gaylord 17/11/1995 6.73i 6.75i BEL Vleminckx Kobe 31/05/1998 6.65i 6.65i BLR Bliznets Dzianis 12/03/1995 6.75i 6.75i BLR Bohdan Maksim 19/03/1997 6.77i 6.77i BLR Zabalotny Yury 24/02/1997 6.72i 6.72i BUL Dimitrov Denis 10/02/1994 6.65i 6.73i BUL Jivkov Vesselin 26/01/2001 6.76i 6.80i CZE Hampl Štěpán 10/11/1999 6.70i 6.70i CZE Stromšík Zdeněk 25/11/1994 6.60i 6.68i CZE Veleba Jan 06/12/1986 6.65i 6.65i DEN Hansen Simon 30/06/1998 6.75i 6.75i DEN Kjær Emil Mader 20/12/1999 6.77i 6.77i DEN Musah Kojo 15/04/1996 6.61i 6.61i ESP López Sergio 05/07/1999 6.67i 6.74i ESP Rodríguez Daniel 26/01/1995 6.67i 6.67i ESP Sanchez Ricardo 10/08/1999 6.75i 6.75i EST Nazarov Karl Erik 17/03/1999 6.63i 6.63i FIN Illukka Riku 21/09/1999 6.73i 6.73i FIN Purola Samuel 19/05/2000 6.67i 6.67i FIN Samuelsson Samuli 23/06/1995 6.66i 6.66i FRA Fall Mouhamadou 25/02/1992 6.62i 6.62i FRA Golitin Amaury 28/01/1997 6.62i 6.62i GBR Aikines-Aryeetey Harry 29/08/1988 6.55i 6.67i GBR Bromby Oliver 30/03/1998 6.63i 6.65i GBR Robertson Andrew 17/12/1990 6.54i 6.61i GER Corucle Philipp 18/07/1997 6.62i -

Surname Fore-Names Date of Death Age Plot No ADAMS CHARLOTTE
Surname Fore-Names Date of Death Age Plot No ADAMS CHARLOTTE 7 November 1885 47 L108 ADAMS ELLEN 22 June 1899 66 L108 ADAMS OLIVER 30 October 1912 77 L108 AHERNE ANNIE 09 February 1942 75 D421 AHERNE ELLEN 02 January 1989 97 D421 AHERNE JOHN 08 January 1927 63 D421 AIKEN HENRY 3 December 1872 46 L388 ALEXANDER ALICE ALICIA 20 January 1886 18 M187 ALEXANDER ELIZA 21 June 1886 70 L1378 ALEXANDER IVOR 17 August 1931 75 L1378 ALEXANDER JOHN 24 November 1895 60 M187 ALEXANDER JOHN DAVID 8 September 1876 0 M187 ALEXANDER MARY 02 March 1927 86 M187 ALEXANDER MARY ELIZA 4 January 1876 32 L1378 ALEXANDER PHILIP THOMAS LLEWELLYN 17 March 1886 0 L374/L395 ALEXANDER THOMAS 09 March 1904 47 L374/L395 ALEXANDER WILLIAM 20 October 1886 70 L1378 ALEXANDER WILLIAM THOMAS 15 August 1876 31 L1378 ALLEN ANN 19 October 1901 84 L1342 ALLEN CHARLES 17 August 1882 65 L1342 ALLEN HENRY 16 June 1876 61 L211 ALLEN MARY ANNE 30 January 1885 75 M439 ALLEN SAMUEL WESLEY 06 October 1920 74 M439 ALLEN SARAH 25 February 1900 81 L211 AMESBURY JAMES 14 September 1911 74 L2871 AMESBURY MARY 04 May 1923 56 L2871 ANDERSON ANNIE 08 January 1909 91 X535 ANDERSON WALTER 17 February 1921 90 X535 ANGEL ELLEN MARIA 14 December 1930 83 L971/L995 ANGEL SARAH 11 March 1898 79 L971/L995 ANGEL WILLIAM 26 January 1922 75 L971/L995 ANGEL WILLIAM 16 March 1898 83 L971/L995 ANGEL WILLIAM ALFRED EDWIN 8 October 1876 L971/L995 ANNING JOHN HENRY 21 Sept 1885 62 L920 ANSTEE HARRIETTE 13 February 1912 75 L1065 ANSTEE JOHN 4 May 1886 56 L1065 ARCH ANN 05 March 1907 62 L882 ARCH ESTHER L882 ARCH -

British Family Names
cs 25o/ £22, Cornrll IBniwwitg |fta*g BOUGHT WITH THE INCOME FROM THE SAGE ENDOWMENT FUND THE GIFT OF Hcnrti W~ Sage 1891 A.+.xas.Q7- B^llll^_ DATE DUE ,•-? AUG 1 5 1944 !Hak 1 3 1^46 Dec? '47T Jan 5' 48 ft e Univeral, CS2501 .B23 " v Llb«"y Brit mii!Sm?nS,£& ori8'" and m 3 1924 olin 029 805 771 The original of this book is in the Cornell University Library. There are no known copyright restrictions in the United States on the use of the text. http://www.archive.org/details/cu31924029805771 BRITISH FAMILY NAMES. : BRITISH FAMILY NAMES ftbetr ©riain ano fIDeaning, Lists of Scandinavian, Frisian, Anglo-Saxon, and Norman Names. HENRY BARBER, M.D. (Clerk), "*• AUTHOR OF : ' FURNESS AND CARTMEL NOTES,' THE CISTERCIAN ABBEY OF MAULBRONN,' ( SOME QUEER NAMES,' ' THE SHRINE OF ST. BONIFACE AT FULDA,' 'POPULAR AMUSEMENTS IN GERMANY,' ETC. ' "What's in a name ? —Romeo and yuliet. ' I believe now, there is some secret power and virtue in a name.' Burton's Anatomy ofMelancholy. LONDON ELLIOT STOCK, 62, PATERNOSTER ROW, E.C. 1894. 4136 CONTENTS. Preface - vii Books Consulted - ix Introduction i British Surnames - 3 nicknames 7 clan or tribal names 8 place-names - ii official names 12 trade names 12 christian names 1 foreign names 1 foundling names 1 Lists of Ancient Patronymics : old norse personal names 1 frisian personal and family names 3 names of persons entered in domesday book as HOLDING LANDS temp. KING ED. CONFR. 37 names of tenants in chief in domesday book 5 names of under-tenants of lands at the time of the domesday survey 56 Norman Names 66 Alphabetical List of British Surnames 78 Appendix 233 PREFACE. -

Distinct Types of Short Open Reading Frames Are Translated in Plant Cells
Downloaded from genome.cshlp.org on September 30, 2021 - Published by Cold Spring Harbor Laboratory Press 1 Distinct types of short open reading frames are translated in 2 plant cells 3 4 Igor Fesenko1,*, Ilya Kirov2, Andrey Kniazev1, Regina Khazigaleeva1, Vassili Lazarev3,4, Daria 5 Kharlampieva3, Ekaterina Grafskaia3,4, Viktor Zgoda5, Ivan Butenko3, Georgy Arapidi1,3, Anna 6 Mamaeva1, Vadim Ivanov1, Vadim Govorun3. 7 1Laboratory of functional genomics and plant proteomics, Shemyakin and Ovchinnikov Institute of 8 Bioorganic Chemistry, Moscow, Russian Federation; 2Laboratory of marker-assisted and genomic 9 selection of plants, All-Russian Research Institute of Agricultural Biotechnology, Moscow, Russian 10 Federation; 3 Research Institute for Physico-Chemical Medicine, Moscow, Russian Federation; 4Moscow 11 Institute of Physics and Technology, Dolgoprudny, Moscow region, Russia; 5Laboratory of System 12 Biology, Institute of Biomedical Chemistry, Moscow, Russian Federation. 13 14 Corresponding author(s). 15 * Igor Fesenko, e-mail: [email protected] 16 17 Running title: Translation of sORFs in moss 18 19 Keywords: short open reading frames, plant peptides, LC-MS/MS, evolution, alternative splicing, 20 lncRNA 21 22 ABSTRACT 23 Genomes contain millions of short (<100 codons) open reading frames (sORFs), which are usually 24 dismissed during gene annotation. Nevertheless, peptides encoded by such sORFs can play important 25 biological roles, and their impact on cellular processes has long been underestimated. Here, we 1 Downloaded from genome.cshlp.org on September 30, 2021 - Published by Cold Spring Harbor Laboratory Press 26 analyzed approximately 70,000 transcribed sORFs in the model plant Physcomitrella patens (moss). 27 Several distinct classes of sORFs that differ in terms of their position on transcripts and the level of 28 evolutionary conservation are present in the moss genome. -

ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation
European Heart Journal (2020) 00,1À126 ESC GUIDELINES doi:10.1093/eurheartj/ehaa612 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in Downloaded from https://academic.oup.com/eurheartj/advance-article/doi/10.1093/eurheartj/ehaa612/5899003 by guest on 31 August 2020 collaboration with the European Association of Cardio-Thoracic Surgery (EACTS) The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC Authors/Task Force Members: Gerhard Hindricks* (Chairperson) (Germany), Tatjana Potpara* (Chairperson) (Serbia), Nikolaos Dagres (Germany), Elena Arbelo (Spain), Jeroen J. Bax (Netherlands), Carina Blomstro¨m-Lundqvist (Sweden), Giuseppe Boriani (Italy), Manuel Castella1 (Spain), Gheorghe-Andrei Dan (Romania), Polychronis E. Dilaveris (Greece), Laurent Fauchier (France), Gerasimos Filippatos (Greece), Jonathan M. Kalman (Australia), Mark La Meir1 * Corresponding authors: The two chairpersons contributed equally to the document. Gerhard Hindricks, University Clinic of Cardiology, Heart Center Leipzig, Department of Cardiology and Electrophysiology, Leipzig Heart Institute, Stru¨mpellstr. 39, 04289 Leipzig, Germany. Tel: þ49 34 1865 1410, Fax: þ49 34 1865 1460, Email: [email protected] Tatjana Potpara, School of Medicine, Belgrade University, dr Subotica 8, 11000 Belgrade, Serbia, and Cardiology Clinic, Clinical Centre of Serbia, -

The SH-SY5Y Cell Line in Parkinson's Disease Research
Xicoy et al. Molecular Neurodegeneration (2017) 12:10 DOI 10.1186/s13024-017-0149-0 REVIEW Open Access The SH-SY5Y cell line in Parkinson’s disease research: a systematic review Helena Xicoy1,2, Bé Wieringa1 and Gerard J.M. Martens2* Abstract Parkinson’s disease (PD) is a devastating and highly prevalent neurodegenerative disease for which only symptomatic treatment is available. In order to develop a truly effective disease-modifying therapy, improvement of our current understanding of the molecular and cellular mechanisms underlying PD pathogenesis and progression is crucial. For this purpose, standardization of research protocols and disease models is necessary. As human dopaminergic neurons, the cells mainly affected in PD, are difficult to obtain and maintain as primary cells, current PD research is mostly performed with permanently established neuronal cell models, in particular the neuroblastoma SH-SY5Y lineage. This cell line is frequently chosen because of its human origin, catecholaminergic (though not strictly dopaminergic) neuronal properties, and ease of maintenance. However, there is no consensus on many fundamental aspects that are associated with its use, such as the effects of culture media composition and of variations in differentiation protocols. Here we present the outcome of a systematic review of scientific articles that have used SH-SY5Y cells to explore PD. We describe the cell source, culture conditions, differentiation protocols, methods/approaches used to mimic PD and the preclinical validation of the SH-SY5Y findings by employing alternative cellular and animal models. Thus, this overview may help to standardize the use of the SH-SY5Y cell line in PD research and serve as a future user’sguide. -

The Latest Guild News and Updates
Vol 9 Issue January–March 2008 Also in this issue... • Focus on a one-name study: did the Vayro surname come with the Spanish Armada? • Rounding up non-conformist registers not in the county record office The world’s leading publication for one-namers All the latest Guild news and updates GUILD OFFICERS CHAIRMAN Box G, 14 Charterhouse Buildings Peter Walker Goswell Road, London EC1M 7BA 24 Bacons Drive Tel: 0800 011 2182 Cuffley Guild information E-mail: [email protected] Hertfordshire Website: www.one-name.org EN6 4DU Registered as a charity in England 01707 873778 Sales and Wales No. 802048 [email protected] AS well as Guild publications, the Sales Manager has a supply of Jour- VICE-CHAIRMAN nal folders, ties, lapel badges and Paul Millington back issues of the Journal. The 58 Belmont Street address is: President Worcester Derek A Palgrave MA FRHistS FSG Worcestershire Howard Benbrook WR3 8NN 7 Amber Hill Vice-Presidents 01905 745217 Camberley John Hebden [email protected] Surrey Richard Moore FSG GU15 1EB Iain Swinnerton SECRETARY England Alec Tritton Kirsty Gray E-mail enquiries to: 11 Brendon Close [email protected] Tilehurst, Reading Berkshire RG30 6EA Guild Committee 0118 941 4833 Forum The Committee consists of the four [email protected] THIS online discussion forum is Officers, plus the following: open to any member with access to TREASURER e-mail. You can join the list by Gordon Adshead Cliff Kemball sending a message with your mem- Peter Copsey 168 Green Lane bership number to: Peter Hagger Chislehurst [email protected] Barbara Harvey Kent BR7 6AY To e-mail a message to the forum, David Mellor 0208 467 8865 send it to: Roy Rayment [email protected] [email protected] Roy Stockdill Ken Toll Regional Representatives Sandra Turner * * * * * * * * * * * * Steven Whitaker The Guild has Regional Reps in many areas. -
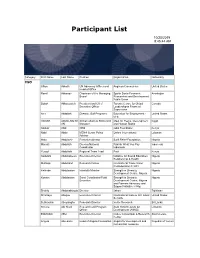
Participant List
Participant List 10/20/2019 8:45:44 AM Category First Name Last Name Position Organization Nationality CSO Jillian Abballe UN Advocacy Officer and Anglican Communion United States Head of Office Ramil Abbasov Chariman of the Managing Spektr Socio-Economic Azerbaijan Board Researches and Development Public Union Babak Abbaszadeh President and Chief Toronto Centre for Global Canada Executive Officer Leadership in Financial Supervision Amr Abdallah Director, Gulf Programs Educaiton for Employment - United States EFE HAGAR ABDELRAHM African affairs & SDGs Unit Maat for Peace, Development Egypt AN Manager and Human Rights Abukar Abdi CEO Juba Foundation Kenya Nabil Abdo MENA Senior Policy Oxfam International Lebanon Advisor Mala Abdulaziz Executive director Swift Relief Foundation Nigeria Maryati Abdullah Director/National Publish What You Pay Indonesia Coordinator Indonesia Yussuf Abdullahi Regional Team Lead Pact Kenya Abdulahi Abdulraheem Executive Director Initiative for Sound Education Nigeria Relationship & Health Muttaqa Abdulra'uf Research Fellow International Trade Union Nigeria Confederation (ITUC) Kehinde Abdulsalam Interfaith Minister Strength in Diversity Nigeria Development Centre, Nigeria Kassim Abdulsalam Zonal Coordinator/Field Strength in Diversity Nigeria Executive Development Centre, Nigeria and Farmers Advocacy and Support Initiative in Nig Shahlo Abdunabizoda Director Jahon Tajikistan Shontaye Abegaz Executive Director International Insitute for Human United States Security Subhashini Abeysinghe Research Director Verite -

University of Dundee MASTER of PHILOSOPHY Changing British Perceptions of Spain in Times of War and Revolution, 1808 to 1838
University of Dundee MASTER OF PHILOSOPHY Changing British Perceptions of Spain in Times of War and Revolution, 1808 to 1838 Holsman, John Robert Award date: 2014 Link to publication General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Download date: 29. Sep. 2021 MASTER OF PHILOSOPHY Changing British Perceptions of Spain in Times of War and Revolution, 1808 to 1838 John Robert Holsman 2014 University of Dundee Conditions for Use and Duplication Copyright of this work belongs to the author unless otherwise identified in the body of the thesis. It is permitted to use and duplicate this work only for personal and non-commercial research, study or criticism/review. You must obtain prior written consent from the author for any other use. Any quotation from this thesis must be acknowledged using the normal academic conventions. It is not permitted to supply the whole or part of this thesis to any other person or to post the same on any website or other online location without the prior written consent of the author. -

Muslims in Spain, 1492–1814 Mediterranean Reconfigurations Intercultural Trade, Commercial Litigation, and Legal Pluralism
Muslims in Spain, 1492– 1814 Mediterranean Reconfigurations Intercultural Trade, Commercial Litigation, and Legal Pluralism Series Editors Wolfgang Kaiser (Université Paris I, Panthéon- Sorbonne) Guillaume Calafat (Université Paris I, Panthéon- Sorbonne) volume 3 The titles published in this series are listed at brill.com/ cmed Muslims in Spain, 1492– 1814 Living and Negotiating in the Land of the Infidel By Eloy Martín Corrales Translated by Consuelo López- Morillas LEIDEN | BOSTON This is an open access title distributed under the terms of the CC BY-NC 4.0 license, which permits any non-commercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited. Further information and the complete license text can be found at https://creativecommons.org/licenses/by-nc/4.0/ The terms of the CC license apply only to the original material. The use of material from other sources (indicated by a reference) such as diagrams, illustrations, photos and text samples may require further permission from the respective copyright holder. Cover illustration: “El embajador de Marruecos” (Catalog Number: G002789) Museo del Prado. Library of Congress Cataloging-in-Publication Data Names: Martín Corrales, E. (Eloy), author. | Lopez-Morillas, Consuelo, translator. Title: Muslims in Spain, 1492-1814 : living and negotiating in the land of the infidel / by Eloy Martín-Corrales ; translated by Consuelo López-Morillas. Description: Leiden ; Boston : Brill, [2021] | Series: Mediterranean reconfigurations ; volume 3 | Original title unknown. | Includes bibliographical references and index. Identifiers: LCCN 2020046144 (print) | LCCN 2020046145 (ebook) | ISBN 9789004381476 (hardback) | ISBN 9789004443761 (ebook) Subjects: LCSH: Muslims—Spain—History. | Spain—Ethnic relations—History. -

Stress-Induced Endogenous Sirnas Targeting Regulatory Intron Sequences in Brachypodium
Downloaded from rnajournal.cshlp.org on October 4, 2021 - Published by Cold Spring Harbor Laboratory Press BIOINFORMATICS Stress-induced endogenous siRNAs targeting regulatory intron sequences in Brachypodium HSIAO-LIN V. WANG, BRANDON L. DINWIDDIE, HERMAN LEE, and JULIA A. CHEKANOVA School of Biological Sciences, University of Missouri–Kansas City, Kansas City, Missouri 64110, USA ABSTRACT Exposure to abiotic stresses triggers global changes in the expression of thousands of eukaryotic genes at the transcriptional and post-transcriptional levels. Small RNA (smRNA) pathways and splicing both function as crucial mechanisms regulating stress-responsive gene expression. However, examples of smRNAs regulating gene expression remain largely limited to effects on mRNA stability, translation, and epigenetic regulation. Also, our understanding of the networks controlling plant gene expression in response to environmental changes, and examples of these regulatory pathways intersecting, remains limited. Here, to investigate the role of smRNAs in stress responses we examined smRNA transcriptomes of Brachypodium distachyon plants subjected to various abiotic stresses. We found that exposure to different abiotic stresses specifically induced a group of novel, endogenous small interfering RNAs (stress-induced, UTR-derived siRNAs, or sutr-siRNAs) that originate from the 3′ UTRs of a subset of coding genes. Our bioinformatics analyses predicted that sutr-siRNAs have potential regulatory functions and that over 90% of sutr-siRNAs target intronic regions of many mRNAs in trans. Importantly, a subgroup of these sutr- siRNAs target the important intron regulatory regions, such as branch point sequences, that could affect splicing. Our study indicates that in Brachypodium, sutr-siRNAs may affect splicing by masking or changing accessibility of specific cis-elements through base-pairing interactions to mediate gene expression in response to stresses.