The Preservation and Protection of Native Biodiversity in The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ehrharta Calycina
Information on measures and related costs in relation to species considered for inclusion on the Union list: Ehrharta calycina This note has been drafted by IUCN within the framework of the contract No 07.0202/2017/763436/SER/ENV.D2 “Technical and Scientific support in relation to the Implementation of Regulation 1143/2014 on Invasive Alien Species”. The information and views set out in this note do not necessarily reflect the official opinion of the Commission, or IUCN. The Commission does not guarantee the accuracy of the data included in this note. Neither the Commission nor IUCN or any person acting on the Commission’s behalf, including any authors or contributors of the notes themselves, may be held responsible for the use which may be made of the information contained therein. Reproduction is authorised provided the source is acknowledged. This document shall be cited as: Visser, V. 2018. Information on measures and related costs in relation to species considered for inclusion on the Union list: Ehrharta calycina. Technical note prepared by IUCN for the European Commission. Date of completion: 25/10/2018 Comments which could support improvement of this document are welcome. Please send your comments by e-mail to [email protected]. Species (scientific name) Ehrharta calycina Sm. Pl. Ic. Ined. t. 33. Species (common name) Perennial veldt grass, purple veldt grass, veldt grass, common ehrharta, gewone ehrharta (Afrikaans), rooisaadgras (Afrikaans). Author(s) Vernon Visser, African Climate & Development Institute Date Completed 25/10/2018 Reviewer Courtenay A. Ray, Arizona State University Summary Highlight of measures that provide the most cost-effective options to prevent the introduction, achieve early detection, rapidly eradicate and manage the species, including significant gaps in information or knowledge to identify cost-effective measures. -

Conceptual Design Documentation
Appendix A: Conceptual Design Documentation APPENDIX A Conceptual Design Documentation June 2019 A-1 APPENDIX A: CONCEPTUAL DESIGN DOCUMENTATION The environmental analyses in the NEPA and CEQA documents for the proposed improvements at Oceano County Airport (the Airport) are based on conceptual designs prepared to provide a realistic basis for assessing their environmental consequences. 1. Widen runway from 50 to 60 feet 2. Widen Taxiways A, A-1, A-2, A-3, and A-4 from 20 to 25 feet 3. Relocate segmented circle and wind cone 4. Installation of taxiway edge lighting 5. Installation of hold position signage 6. Installation of a new electrical vault and connections 7. Installation of a pollution control facility (wash rack) CIVIL ENGINEERING CALCULATIONS The purpose of this conceptual design effort is to identify the amount of impervious surface, grading (cut and fill) and drainage implications of the projects identified above. The conceptual design calculations detailed in the following figures indicate that Projects 1 and 2, widening the runways and taxiways would increase the total amount of impervious surface on the Airport by 32,016 square feet, or 0.73 acres; a 6.6 percent increase in the Airport’s impervious surface area. Drainage patterns would remain the same as both the runway and taxiways would continue to sheet flow from their centerlines to the edge of pavement and then into open, grassed areas. The existing drainage system is able to accommodate the modest increase in stormwater runoff that would occur, particularly as soil conditions on the Airport are conducive to infiltration. Figure A-1 shows the locations of the seven projects incorporated in the Proposed Action. -

Montaña De Oro Checklist-07Jun19
Checklist1 of Vascular Flora of Montaña de Oro State Park San Luis Obispo County, California (07 June 2019) David J. Keil Robert F. Hoover Herbarium Biological Sciences Department California Polytechnic State University San Luis Obispo, California Scientific Name Common Name Family Rare n Abronia latifolia yellow sand-verbena NYCTAGINACEAE v n Abronia maritima beach sand-verbena, red NYCTAGINACEAE 4.2 v sand-verbena n Abronia umbellata var. umbellata purple sand-verbena NYCTAGINACEAE v n Acer macrophyllum big-leaf maple SAPINDACEAE v n ❀ Achillea millefolium yarrow ASTERACEAE v n Acmispon brachycarpus shortpod deervetch FABACEAE v 1 Please notify the author of additions or corrections to this list ([email protected]). ❀ — See Wildflowers of San Luis Obispo, California, second edition (2018) for photograph. Most are illustrated in the first edition as well; old names for some species in square brackets. n — California native n1 — California native but planted at Montaña de Oro. i — exotic species, introduced to California, naturalized or waif. v — documented by one or more specimens (Consortium of California Herbaria record; specimen in OBI; or collection that has not yet been accessioned) o — observed during field surveys; no voucher specimen known R—California Rare Plant Rank Scientific Name Common Name Family Rare n ❀ Acmispon glaber var. glaber common deerweed FABACEAE v n Acmispon heermannii var. orbicularis woolly deer-vetch FABACEAE v n Acmispon junceus var. biolettii Biolett's rush deerweed FABACEAE v n Acmispon junceus var. junceus common rush deerweed FABACEAE v n Acmispon maritimus var. maritimus coastal deer-vetch FABACEAE v n Acmispon micranthus fishhook deervetch FABACEAE v n Acmispon parviflorus miniature deervetch FABACEAE o n ❀ Acmispon strigosus strigose deer-vetch FABACEAE v n Actaea rubra baneberry RANUNCULACEAE v n ❀ Adelinia grandis Pacific hound's tongue BORAGINACEAE v n ❀ Adenostoma fasciculatum var. -
BFS048 Site Species List
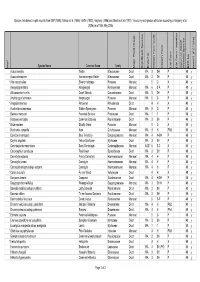
Species lists based on plot records from DEP (1996), Gibson et al. (1994), Griffin (1993), Keighery (1996) and Weston et al. (1992). Taxonomy and species attributes according to Keighery et al. (2006) as of 16th May 2005. Species Name Common Name Family Major Plant Group Significant Species Endemic Growth Form Code Growth Form Life Form Life Form - aquatics Common SSCP Wetland Species BFS No kens01 (FCT23a) Wd? Acacia sessilis Wattle Mimosaceae Dicot WA 3 SH P 48 y Acacia stenoptera Narrow-winged Wattle Mimosaceae Dicot WA 3 SH P 48 y * Aira caryophyllea Silvery Hairgrass Poaceae Monocot 5 G A 48 y Alexgeorgea nitens Alexgeorgea Restionaceae Monocot WA 6 S-R P 48 y Allocasuarina humilis Dwarf Sheoak Casuarinaceae Dicot WA 3 SH P 48 y Amphipogon turbinatus Amphipogon Poaceae Monocot WA 5 G P 48 y * Anagallis arvensis Pimpernel Primulaceae Dicot 4 H A 48 y Austrostipa compressa Golden Speargrass Poaceae Monocot WA 5 G P 48 y Banksia menziesii Firewood Banksia Proteaceae Dicot WA 1 T P 48 y Bossiaea eriocarpa Common Bossiaea Papilionaceae Dicot WA 3 SH P 48 y * Briza maxima Blowfly Grass Poaceae Monocot 5 G A 48 y Burchardia congesta Kara Colchicaceae Monocot WA 4 H PAB 48 y Calectasia narragara Blue Tinsel Lily Dasypogonaceae Monocot WA 4 H-SH P 48 y Calytrix angulata Yellow Starflower Myrtaceae Dicot WA 3 SH P 48 y Centrolepis drummondiana Sand Centrolepis Centrolepidaceae Monocot AUST 6 S-C A 48 y Conostephium pendulum Pearlflower Epacridaceae Dicot WA 3 SH P 48 y Conostylis aculeata Prickly Conostylis Haemodoraceae Monocot WA 4 H P 48 y Conostylis juncea Conostylis Haemodoraceae Monocot WA 4 H P 48 y Conostylis setigera subsp. -

Poaceae: Pooideae) Based on Plastid and Nuclear DNA Sequences
d i v e r s i t y , p h y l o g e n y , a n d e v o l u t i o n i n t h e monocotyledons e d i t e d b y s e b e r g , p e t e r s e n , b a r f o d & d a v i s a a r h u s u n i v e r s i t y p r e s s , d e n m a r k , 2 0 1 0 Phylogenetics of Stipeae (Poaceae: Pooideae) Based on Plastid and Nuclear DNA Sequences Konstantin Romaschenko,1 Paul M. Peterson,2 Robert J. Soreng,2 Núria Garcia-Jacas,3 and Alfonso Susanna3 1M. G. Kholodny Institute of Botany, Tereshchenkovska 2, 01601 Kiev, Ukraine 2Smithsonian Institution, Department of Botany MRC-166, National Museum of Natural History, P.O. Box 37012, Washington, District of Columbia 20013-7012 USA. 3Laboratory of Molecular Systematics, Botanic Institute of Barcelona (CSIC-ICUB), Pg. del Migdia, s.n., E08038 Barcelona, Spain Author for correspondence ([email protected]) Abstract—The Stipeae tribe is a group of 400−600 grass species of worldwide distribution that are currently placed in 21 genera. The ‘needlegrasses’ are char- acterized by having single-flowered spikelets and stout, terminally-awned lem- mas. We conducted a molecular phylogenetic study of the Stipeae (including all genera except Anemanthele) using a total of 94 species (nine species were used as outgroups) based on five plastid DNA regions (trnK-5’matK, matK, trnHGUG-psbA, trnL5’-trnF, and ndhF) and a single nuclear DNA region (ITS). -

Morro Creek Natural Environment Study
Morro Creek Multi-Use Trail and Bridge Project Natural Environment Study San Luis Obispo County, California Federal Project Number CASB12RP-5391(013) MB-2013-S2 05-SLO-0-MOBY December 2013 For individuals with sensory disabilities, this document is available in Braille, large print, on audiocassette, or computer disk. To obtain a copy in one of these alternate formats, please call or write to Caltrans, Attn: Brandy Rider, Caltrans District 5 Environmental Stewardship Branch, 50 Higuera Street, San Luis Obispo, CA 93401; 805-549-3182 Voice, or use the California Relay Service TTY number, 805-549-3259. This page is intentionally left blank. Summary Summary The City of Morro Bay is extending the existing Harborwalk with continuation of a paved pedestrian boardwalk and separate Class I bike path from the existing parking area and crossing on Embarcadero Avenue northward. The City also proposes to install a clear-span pre-engineered/pre-fabricated bike and pedestrian bridge over Morro Creek to connect to north Morro Bay on Embarcadero Road/State Route 41. In addition, the project will include improvements to beach access from the trail, and two interpretive sign stations that will display educational and other information about the cultural and natural history of the region. This project is receiving funding from the Federal Highway Administration (FHWA) and with assistance from Caltrans. As part of its NEPA assignment of federal responsibilities by the FHWA, effective October 1, 2012 and pursuant to 23 USC 326, Caltrans is acting as the lead federal agency for Section 7 of the federal Endangered Species Act. -

Unassisted Invasions: Understanding and Responding to Australia's High
CSIRO PUBLISHING Australian Journal of Botany, 2017, 65, 678–690 Turner Review No. 21 https://doi.org/10.1071/BT17152 Unassisted invasions: understanding and responding to Australia’s high-impact environmental grass weeds Rieks D. van Klinken A,C and Margaret H. Friedel B ACSIRO, EcoSciences Precinct, PO Box 2583, Brisbane, Qld 4001, Australia. BCSIRO, PO Box 2114, Alice Springs, NT 0871, Australia. CCorresponding author. Email: [email protected] Abstract. Alien grass species have been intentionally introduced into Australia since European settlement over 200 years ago, with many subsequently becoming weeds of natural environments. We have identified the subset of these weeds that have invaded and become dominant in environmentally important areas in the absence of modern anthropogenic disturbance, calling them ‘high-impact species’. We also examined why these high-impact species were successful, and what that might mean for management. Seventeen high-impact species were identified through literature review and expert advice; all had arrived by 1945, and all except one were imported intentionally, 16 of the 17 were perennial and four of the 17 were aquatic. They had become dominant in diverse habitats and climates, although some environments still remain largely uninvaded despite apparently ample opportunities. Why these species succeeded remains largely untested, but evidence suggests a combination of ecological novelty (both intended at time of introduction and unanticipated), propagule pressure (through high reproductive rate and dominance in nearby anthropogenically-disturbed habitats) and an ability to respond to, and even alter, natural disturbance regimes (especially fire and inundation). Serious knowledge gaps remain for these species, but indications are that resources could be better focused on understanding and managing this limited group of high-impact species. -

The Effect of Fire and Grazing on the Cumberland Plain Woodlands Samantha Clarke University of Wollongong
University of Wollongong Research Online University of Wollongong Thesis Collection University of Wollongong Thesis Collections 2004 The effect of fire and grazing on the Cumberland Plain Woodlands Samantha Clarke University of Wollongong Recommended Citation Clarke, Samantha, The effect of fire and grazing on the Cumberland Plain Woodlands, Master of Science - Research thesis, School of Biological Sciences, University of Wollongong, 2004. http://ro.uow.edu.au/theses/2700 Research Online is the open access institutional repository for the University of Wollongong. For further information contact the UOW Library: [email protected] The Effect of Fire and Grazing on the Cumberland Plain Woodlands A thesis submitted in partial fulfillment of the requirements for the award of the degree Master of Science (Research) from THE UNIVERSITY OF WOLLONGONG By SAMANTHA CLARKE Bachelor of Science (Biology) DEPARTMENT OF BIOLOGICAL SCIENCES 2004 CERTIFICATION I, Samantha Clarke, declare that this thesis, submitted in partial fulfillment of the requirements for the award of Master of Science (Research), in the Department of Biological Sciences, University of Wollongong, is wholly my own work unless otherwise referenced or acknowledged. The document has not been submitted for qualifications at any other academic institution. Samantha Clarke 20 June 2004 ABSTRACT Temperate grassy woodlands throughout the world have suffered the effects of changed disturbance regimes, in particular, fire and grazing, due to human activities. Since European settlement fire and tree clearing has been used to modify grassy woodland vegetation for livestock grazing and agriculture. As a consequence some species, particularly shrubs and trees, have been reduced or eliminated and both native and introduced grasses have become more dominant. -

Systematic Studies of Australian Stipoid Grasses (Austrostipa) Based on Micro-Morphological and Molecular Characteristics
BIODIVERSITAS ISSN: 1412-033X (printed edition) Volume 11, Number 1, January 2010 ISSN: 2085-4722 (electronic) Pages: 9-14 DOI: 10.13057/biodiv/d110103 Systematic studies of Australian stipoid grasses (Austrostipa) based on micro-morphological and molecular characteristics BETTY MAULIYA BUSTAM♥ Biology Department, Faculty of Mathematics and Natural Sciences, Syiah Kuala University, Jl. Syech Abdur Rauf 3, Darussalam, Banda Aceh 23111, Aceh, Indonesia, Tel./fax. +62-651-7428212, +62-651-7410248, email for correspondence: [email protected]. Manuscript received: 25 October 2009. Revision accepted: 26 December 2009. ABSTRACT Bustam BM (2010) Systematic studies of Australian stipoid grasses (Austrostipa) based on micro-morphological and molecular characteristics. Biodiversitas 11: 9-14. This research is one of many studies on stipoid grasses organized by the International Stipeae Working Group (ISWG). This research tested the subgeneric classification of Austrostipa proposed by Jacobs and Everett (1996) and tested how informative the micro morphological characters used. Data were collected from herbarium specimens of 36 species (33 species of Austrostipa, two species of Hesperostipa and one species of Anemanthele) at Royal Botanic Gardens, Sydney. Twenty eight micro morphological characters were used. The data were collected from both adaxial and abaxial surfaces of leaves, and from the lemma epidermis using a scanning electron microscope (SEM). ISWG provided the molecular data. Parsimony analysis and a distance method (Unweighteic Pair Group with Arithmatic Mean: UPGMA) were used to analyze micro-morphological and molecular data separately. Only UPGMA analysis was used to analyze the combined data. The results support the monophyly of Austrostipa. However, there is a little support for the subgeneric classification of Austrostipa proposed by Jacobs and Everett (1996), other than for the consistent recognition of Falcatae. -

A Checklist of Vascular Plants Endemic to California
Humboldt State University Digital Commons @ Humboldt State University Botanical Studies Open Educational Resources and Data 3-2020 A Checklist of Vascular Plants Endemic to California James P. Smith Jr Humboldt State University, [email protected] Follow this and additional works at: https://digitalcommons.humboldt.edu/botany_jps Part of the Botany Commons Recommended Citation Smith, James P. Jr, "A Checklist of Vascular Plants Endemic to California" (2020). Botanical Studies. 42. https://digitalcommons.humboldt.edu/botany_jps/42 This Flora of California is brought to you for free and open access by the Open Educational Resources and Data at Digital Commons @ Humboldt State University. It has been accepted for inclusion in Botanical Studies by an authorized administrator of Digital Commons @ Humboldt State University. For more information, please contact [email protected]. A LIST OF THE VASCULAR PLANTS ENDEMIC TO CALIFORNIA Compiled By James P. Smith, Jr. Professor Emeritus of Botany Department of Biological Sciences Humboldt State University Arcata, California 13 February 2020 CONTENTS Willis Jepson (1923-1925) recognized that the assemblage of plants that characterized our flora excludes the desert province of southwest California Introduction. 1 and extends beyond its political boundaries to include An Overview. 2 southwestern Oregon, a small portion of western Endemic Genera . 2 Nevada, and the northern portion of Baja California, Almost Endemic Genera . 3 Mexico. This expanded region became known as the California Floristic Province (CFP). Keep in mind that List of Endemic Plants . 4 not all plants endemic to California lie within the CFP Plants Endemic to a Single County or Island 24 and others that are endemic to the CFP are not County and Channel Island Abbreviations . -

Ecology of the Ecological Community
APPENDIX A: BIOLOGY AND ECOLOGY OF THE ECOLOGICAL COMMUNITY This appendix provides further relevant information about the biology and ecology of the KI Narrow-leaved Mallee Woodland ecological community to better understand what the ecological community is and how it functions. Biology of key species The dominant presence of Eucalyptus cneorifolia in the tree canopy is a diagnostic feature of the ecological community. The KI narrow-leaved mallee is an upright tree, to ten metres tall, sometimes with a single stem or in the form of a multi-stemmed mallee (Brooker et al., 2002). It generally forms a dense canopy of narrow, dark green leaves. The leaves have conspicuous oil glands and the species was used for the Eucalyptus oil industry (Willoughby et al., 2001). The KI narrow-leaved mallee develops a lignotuber at the base of the trunk. Regeneration after disturbances, such as fire, occurs mostly through resprouts from the lignotuber. Regeneration from canopy-stored seed or from the soil seed bank is thought to be limited (Rawson et al., 2012). Other plant species may be widespread throughout the ecological community, for instance Melaleuca uncinata (broombrush), M. lanceolata (dryland tea-tree) or Thryptomene ericaea (heath thryptomene) are common at many sites. However, no other plant species occurs across all sites nor has such an obvious presence that it governs the structure and appearance of the ecological community. Similar considerations apply to the faunal component of the ecological community. The upper height limit for mallee eucalypts is typically about 9 metres (Parsons, 1994). An unusual feature of mallees on KI is that they can be taller, sometimes exceptionally so. -

Molecular Phylogenetics and Micromorphology of Australasian
Edinburgh Research Explorer Molecular phylogenetics and micromorphology of Australasian stipeae (poaceae, subfamily pooideae), and the interrelation of whole-genome duplication and evolutionary radiations in this grass tribe Citation for published version: Tkach, N, Nobis, M, Schneider, J, Becher, H, Winterfeld, G, Jacobs, SWL & Röser, M 2021, 'Molecular phylogenetics and micromorphology of Australasian stipeae (poaceae, subfamily pooideae), and the interrelation of whole-genome duplication and evolutionary radiations in this grass tribe', Frontiers in plant science, vol. 11. https://doi.org/10.3389/fpls.2020.630788 Digital Object Identifier (DOI): 10.3389/fpls.2020.630788 Link: Link to publication record in Edinburgh Research Explorer Document Version: Publisher's PDF, also known as Version of record Published In: Frontiers in plant science General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 09. Oct. 2021 fpls-11-630788