ESCMID Online Lecture Library @ by Author
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fig. L COMPOSITIONS and METHODS to INHIBIT STEM CELL and PROGENITOR CELL BINDING to LYMPHOID TISSUE and for REGENERATING GERMINAL CENTERS in LYMPHATIC TISSUES
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date Χ 23 February 2012 (23.02.2012) WO 2U12/U24519ft ft A2 (51) International Patent Classification: AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, A61K 31/00 (2006.01) CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (21) International Application Number: HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, PCT/US201 1/048297 KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, (22) International Filing Date: ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, 18 August 201 1 (18.08.201 1) NO, NZ, OM, PE, PG, PH, PL, PT, QA, RO, RS, RU, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, (25) Filing Language: English TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, (26) Publication Language: English ZW. (30) Priority Data: (84) Designated States (unless otherwise indicated, for every 61/374,943 18 August 2010 (18.08.2010) US kind of regional protection available): ARIPO (BW, GH, 61/441,485 10 February 201 1 (10.02.201 1) US GM, KE, LR, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, 61/449,372 4 March 201 1 (04.03.201 1) US ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, (72) Inventor; and EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, ΓΓ, LT, LU, (71) Applicant : DEISHER, Theresa [US/US]; 1420 Fifth LV, MC, MK, MT, NL, NO, PL, PT, RO, RS, SE, SI, SK, Avenue, Seattle, WA 98101 (US). -

Remarkably Similar CTLA-4 Binding Properties of Therapeutic Ipilimumab and Tremelimumab Antibodies
www.impactjournals.com/oncotarget/ Oncotarget, 2017, Vol. 8, (No. 40), pp: 67129-67139 Research Paper Remarkably similar CTLA-4 binding properties of therapeutic ipilimumab and tremelimumab antibodies Mengnan He1,2,*, Yan Chai1,*, Jianxun Qi1, Catherine W.H. Zhang3, Zhou Tong4, Yi Shi1, Jinghua Yan4, Shuguang Tan1 and George F. Gao1,2 1CAS Key Laboratory of Pathogenic Microbiology and Immunology, Institute of Microbiology, Chinese Academy of Sciences, Beijing 100101, China 2University of Chinese Academy of Sciences, Beijing 100049, China 3ImmuFuCell Biotechnology Co., Ltd., Beijing 100102, China 4CAS Key Laboratory of Microbial Physiological and Metabolic Engineering, Institute of Microbiology, Chinese Academy of Sciences, Beijing 100101, China *These authors have contributed equally to this work Correspondence to: George F. Gao, email: [email protected] Shuguang Tan, email: [email protected] Keywords: ipilimumab, CTLA-4, complex structure, tremelimumab Received: April 04, 2017 Accepted: April 21, 2017 Published: May 19, 2017 Copyright: He et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC BY 3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. ABSTRACT Monoclonal antibody based immune checkpoint blockade therapies have achieved clinical successes in management of malignant tumors. As the first monoclonal antibody targeting immune checkpoint molecules entered into clinics, the molecular basis of ipilimumab-based anti-CTLA-4 blockade has not yet been fully understood. In the present study, we report the complex structure of ipilimumab and CTLA-4. The complex structure showed similar contributions from VH and VL of ipilimumab in binding to CTLA-4 front β-sheet strands. -

Predictive QSAR Tools to Aid in Early Process Development of Monoclonal Antibodies
Predictive QSAR tools to aid in early process development of monoclonal antibodies John Micael Andreas Karlberg Published work submitted to Newcastle University for the degree of Doctor of Philosophy in the School of Engineering November 2019 Abstract Monoclonal antibodies (mAbs) have become one of the fastest growing markets for diagnostic and therapeutic treatments over the last 30 years with a global sales revenue around $89 billion reported in 2017. A popular framework widely used in pharmaceutical industries for designing manufacturing processes for mAbs is Quality by Design (QbD) due to providing a structured and systematic approach in investigation and screening process parameters that might influence the product quality. However, due to the large number of product quality attributes (CQAs) and process parameters that exist in an mAb process platform, extensive investigation is needed to characterise their impact on the product quality which makes the process development costly and time consuming. There is thus an urgent need for methods and tools that can be used for early risk-based selection of critical product properties and process factors to reduce the number of potential factors that have to be investigated, thereby aiding in speeding up the process development and reduce costs. In this study, a framework for predictive model development based on Quantitative Structure- Activity Relationship (QSAR) modelling was developed to link structural features and properties of mAbs to Hydrophobic Interaction Chromatography (HIC) retention times and expressed mAb yield from HEK cells. Model development was based on a structured approach for incremental model refinement and evaluation that aided in increasing model performance until becoming acceptable in accordance to the OECD guidelines for QSAR models. -

RT+IO Therapy in NSCLC Draft 4 Clinical Cancer Research 1
Author Manuscript Published OnlineFirst on June 26, 2018; DOI: 10.1158/1078-0432.CCR-17-3620 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. RT+IO Therapy in NSCLC Draft 4 Clinical Cancer Research Title: The Integration of Radiotherapy With Immunotherapy for the Treatment of Non-Small Cell Lung Cancer Running title: Radiotherapy and Immunotherapy in Non-Small Cell Lung Cancer Eric C. Ko1, David Raben2, Silvia C. Formenti1 1Department of Radiation Oncology, Weill Cornell Medicine, New York, New York 2Department of Radiation Oncology, University of Colorado Anschutz Medical Campus, Aurora, Colorado Corresponding Author: Silvia C. Formenti, New York-Presbyterian/Weill Cornell Medicine, 525 East 68th Street, N-046, Box 169, New York, NY 10065-4885; Phone: 212-746-3608; Fax: 212-746-8850; E-mail: [email protected]. Confirmed Target Journal: Clinical Cancer Research Journal Specs (Review Article): Word Count (limit 3750 words): 4090 Abstract Word Count (unstructured, limit ≤250 words): 209 Number of References (≤75): 79 Number of Figures/Tables (5): 1 table, 3 figures 1 Downloaded from clincancerres.aacrjournals.org on September 24, 2021. © 2018 American Association for Cancer Research. Author Manuscript Published OnlineFirst on June 26, 2018; DOI: 10.1158/1078-0432.CCR-17-3620 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. RT+IO Therapy in NSCLC Draft 4 Clinical Cancer Research Abstract Five-year survival rates for non-small cell lung cancer (NSCLC) range from 14% to 49% for stage I to stage IIIA disease, and are <5% for stage IIIB/IV disease. -

New Biological Therapies: Introduction to the Basis of the Risk of Infection
New biological therapies: introduction to the basis of the risk of infection Mario FERNÁNDEZ RUIZ, MD, PhD Unit of Infectious Diseases Hospital Universitario “12 de Octubre”, Madrid ESCMIDInstituto de Investigación eLibraryHospital “12 de Octubre” (i+12) © by author Transparency Declaration Over the last 24 months I have received honoraria for talks on behalf of • Astellas Pharma • Gillead Sciences • Roche • Sanofi • Qiagen Infections and biologicals: a real concern? (two-hour symposium): New biological therapies: introduction to the ESCMIDbasis of the risk of infection eLibrary © by author Paul Ehrlich (1854-1915) • “side-chain” theory (1897) • receptor-ligand concept (1900) • “magic bullet” theory • foundation for specific chemotherapy (1906) • Nobel Prize in Physiology and Medicine (1908) (together with Metchnikoff) Infections and biologicals: a real concern? (two-hour symposium): New biological therapies: introduction to the ESCMIDbasis of the risk of infection eLibrary © by author 1981: B-1 antibody (tositumomab) anti-CD20 monoclonal antibody 1997: FDA approval of rituximab for the treatment of relapsed or refractory CD20-positive NHL 2001: FDA approval of imatinib for the treatment of chronic myelogenous leukemia Infections and biologicals: a real concern? (two-hour symposium): New biological therapies: introduction to the ESCMIDbasis of the risk of infection eLibrary © by author Functional classification of targeted (biological) agents • Agents targeting soluble immune effector molecules • Agents targeting cell surface receptors -

Timmerman Et Al-2020-Urelumab
Urelumab alone or in combination with rituximab in patients with relapsed or refractory B-cell lymphoma John Timmerman, Charles Herbaux, Vincent Ribrag, Andrew D. Zelenetz, Roch Houot, Sattva S. Neelapu, Theodore Logan, Izidore S. Lossos, Walter Urba, Gilles Salles, et al. To cite this version: John Timmerman, Charles Herbaux, Vincent Ribrag, Andrew D. Zelenetz, Roch Houot, et al.. Ure- lumab alone or in combination with rituximab in patients with relapsed or refractory B-cell lymphoma. American Journal of Hematology, Wiley, 2020, 95 (5), pp.510-520. 10.1002/ajh.25757. hal-02798009 HAL Id: hal-02798009 https://hal-univ-rennes1.archives-ouvertes.fr/hal-02798009 Submitted on 23 Jun 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License Received: 25 October 2019 Revised: 30 January 2020 Accepted: 5 February 2020 DOI: 10.1002/ajh.25757 RESEARCH ARTICLE Urelumab alone or in combination with rituximab in patients with relapsed or refractory B-cell lymphoma John Timmerman1 | Charles -

Immune Checkpoint Inhibition in DLBCL Immunotherapy: “The Cure Is Inside Us”
Mariano Provencio Servicio de Oncología Médica Hospital Universitario Puerta de Hierro Immune checkpoint inhibition in DLBCL Immunotherapy: “The Cure is Inside Us” § Our immune system prevents or limit infections by foreign antigens expressed in microorganisms (bacteria, viruses, etc.) § Our immune system can also recognize and destroy cancer cells….. • However, cancer cells have developed “escape mechanisms” to avoid their destruction by immune cells…“put the brakes on” • Immuno-Oncology: Find ways of “unleashing” the power of our body’s immune system to treat or prevent cancer…T-lymphocytes (T- cells) Detectives Dendritic cells Killer –T cells Microenvironment antigen (flags) Detectives Dendritic cells Killer –T cells Tumor infiltranting T cell recognizable ags Cor e Algorithms Margin Neo-antigens antigen (flags) 5 6 Scott DW et al. Nature Rev 2014 Strategy approach § Effective immune response: barriers § microenviroment § Activate anti-tumor immune response § inhibitory receptors: blocking antibodies § Nivo, Pembro, Ipi,…(anti PD 1) (CTLA4) § combining 2 checkpoint inhibitors § combining with chemotherapy § activate receptors: agonist § Urelumab § Utumilumab § Varlilumab Strategy approach § Effective immune response: barriers § microenviroment Inactivated effector T cell angiogenesis metabolism Strategy approach PDL-1 Effective immune CTLA-4 response: barriers Lymphoma PDL-1 PD-1 PDL-1 TIM-3 PDL-1 PDL-1 mTOR LAG-3 OXPHOS MHC1 Aerobic glycolysis Interferon gamma EB virus T cell activation antigen presenting cells Strategy approach § -
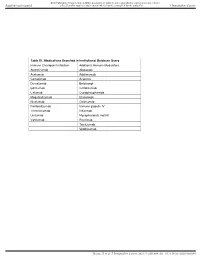
Table S1. Medications Searched in Institutional Database Query Immune Checkpoint Inhibitors Additional Immune Modulators Atezol
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) J Immunother Cancer Table S1. Medications Searched in Institutional Database Query Immune Checkpoint Inhibitors Additional Immune Modulators Atezolizumab Abatacept Avelumab Adalimumab Cemiplimab Anakinra Durvalumab Belatacept Ipilimumab Certolizumab Lirilumab Cyclophosphamide Mogamulizumab Etanercept Nivolumab Golimumab Pembrolizumab Immune globulin IV Tremelimumab Infliximab Urelumab Mycophenolate mofetil Varlilumab Rituximab Tocilizumab Vedolizumab Beattie J, et al. J Immunother Cancer 2021; 9:e001884. doi: 10.1136/jitc-2020-001884 BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) J Immunother Cancer Table S2. Toxicity of additional immune modulators Treatment detail Toxicity Patient 1 day 18,32 Infliximab dosed day 59 P.aeruginosa, S.marcescens pneumonia; died Patient 2 day 9 Infliximab dosed day 44 Febrile neutropenia; P. aeruginosa SBP and day 26 Mycophenolate initiated sepsis; C. albicans fungemia; treated and discharged Patient 8 day 4,11 Infliximab dosed day 14 Disseminated HSV-1; died Patient 26 day 79-128 Infliximab dosed (x7) day 130 E. faecalis, P. aeruginosa bacteremia; died; day 81, 97 Cyclophosphamide dosed Fungal pneumonia on autopsy Beattie J, et al. J Immunother Cancer 2021; 9:e001884. doi: 10.1136/jitc-2020-001884 BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) J Immunother Cancer Table S3. -

Looking for Therapeutic Antibodies in Next Generation Sequencing Repositories
bioRxiv preprint doi: https://doi.org/10.1101/572958; this version posted March 10, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Title: Looking for Therapeutic Antibodies in Next Generation Sequencing Repositories. Authors: Konrad Krawczyk1*, Matthew Raybould2, Aleksandr Kovaltsuk2, Charlotte M. Deane2 1 NaturalAntibody, Hamburg, Germany 2 Oxford University Department of Statistics, Oxford, UK *Correspondence to [email protected] Abstract: Recently it has become possible to query the great diversity of natural antibody repertoires using Next Generation Sequencing (NGS). These methods are capable of producing millions of sequences in a single experiment. Here we compare Clinical Stage Therapeutic antibodies to the ~1b sequences from 60 independent sequencing studies in the Observed Antibody Space Database. Of the 242 post Phase I antibodies, we find 16 with sequence identity matches of 95% or better for both heavy and light chains. There are also 54 perfect matches to therapeutic CDR-H3 regions in the NGS outputs, suggesting a nontrivial amount of convergence between naturally observed sequences and those developed artificially. This has potential implications for both the discovery of antibody therapeutics and the legal protection of commercial antibodies. Introduction Antibodies are proteins in jawed vertebrates that recognize noxious molecules (antigens) for elimination. An organism expresses millions of diverse antibodies to increase the chances that some of them will be able to bind the foreign antigen, initiating the adaptive immune response. -

Tables-Of-Phase-3-Mabs.Pdf
Table 3. Monoclonal antibodies in Phase 2/3 or 3 clinical studies for cancer indications Most advanced Primary sponsoring company INN or code name Molecular format Target(s) phase Phase 3 indications Therapeutic area Janssen Research & Development, LLC JNJ-56022473 Humanized mAb CD123 Phase 2/3 Acute myeloid leukemia Cancer Murine IgG1, Actinium Pharmaceuticals Iomab-B radiolabeled CD45 Phase 3 Acute myeloid leukemia Cancer Humanized IgG1, Seattle Genetics Vadastuximab talirine ADC CD33 Phase 3 Acute myeloid leukemia Cancer TG Therapeutics Ublituximab Chimeric IgG1 CD20 Phase 3 Chronic lymphocytic leukemia Cancer Xencor XMAB-5574, MOR208 Humanized IgG1 CD19 Phase 2/3 Diffuse large B-cell lymphoma Cancer Moxetumomab Murine IgG1 dsFv, AstraZeneca/MedImmune LLC pasudotox immunotoxin CD22 Phase 3 Hairy cell leukemia Cancer Humanized scFv, Viventia Bio Oportuzumab monatox immunotoxin EpCAM Phase 3 Bladder cancer Cancer scFv-targeted liposome containing Merrimack Pharmaceuticals MM-302 doxorubicin HER2 Phase 2/3 Breast cancer Cancer MacroGenics Margetuximab Chimeric IgG1 HER2 Phase 3 Breast cancer Cancer Gastric cancer or gastroesophageal junction Gilead Sciences GS-5745 Humanized IgG4 MMP9 Phase 3 adenocarcinoma; ulcerative colitis (Phase 2/3) Cancer; Immune-mediated disorders Depatuxizumab Humanized IgG1, AbbVie mafodotin ADC EGFR Phase 2/3 Glioblastoma Cancer AstraZeneca/MedImmune LLC Tremelimumab Human IgG2 CTLA4 Phase 3 NSCLC, head & neck cancer, bladder cancer Cancer NSCLC, head & neck cancer, bladder cancer, breast AstraZeneca/MedImmune -

Immunfarmakológia Immunfarmakológia
Gergely: Immunfarmakológia Immunfarmakológia Prof Gergely Péter Az immunpatológiai betegségek döntő többsége gyulladásos, és ennek következtében általában szövetpusztulással járó betegség, melyben – jelenleg – a terápia alapvetően a gyulladás csökkentésére és/vagy megszűntetésére irányul. Vannak kizárólag gyulladásgátló gyógyszereink és vannak olyanok, amelyek az immunreakció(k) bénításával (=immunszuppresszió révén) vagy emellett vezetnek a gyulladás mérsékléséhez. Mind szerkezetileg, mind hatástanilag igen sokféle csoportba oszthatók, az alábbi felosztás elsősorban didaktikus célokat szolgál. 1. Nem-szteroid gyulladásgátlók (‘nonsteroidal antiinflammatory drugs’ NSAID) 2. Kortikoszteroidok 3. Allergia-elleni szerek (antiallergikumok) 4. Sejtoszlás-gátlók (citosztatikumok) 5. Nem citosztatikus hatású immunszuppresszív szerek 6. Egyéb gyulladásgátlók és immunmoduláns szerek 7. Biológiai terápia 1. Nem-szteroid gyulladásgátlók (NSAID) Ezeket a vegyületeket, melyek őse a szalicilsav (jelenleg, mint acetilszalicilsav ‘aszpirin’ használatos), igen kiterjedten alkalmazzák a reumatológiában, az onkológiában és az orvostudomány szinte minden ágában, ahol fájdalom- és lázcsillapításra van szükség. Egyes felmérések szerint a betegek egy ötöde szed valamilyen NSAID készítményt. Szerkezetük alapján a készítményeket több csoportba sorolhatjuk: szalicilátok (pl. acetilszalicilsav) pyrazolidinek (pl. fenilbutazon) ecetsav származékok (pl. indometacin) fenoxiecetsav származékok (pl. diclofenac, aceclofenac)) oxicamok (pl. piroxicam, meloxicam) propionsav -

The Two Tontti Tudiul Lui Hi Ha Unit
THETWO TONTTI USTUDIUL 20170267753A1 LUI HI HA UNIT ( 19) United States (12 ) Patent Application Publication (10 ) Pub. No. : US 2017 /0267753 A1 Ehrenpreis (43 ) Pub . Date : Sep . 21 , 2017 ( 54 ) COMBINATION THERAPY FOR (52 ) U .S . CI. CO - ADMINISTRATION OF MONOCLONAL CPC .. .. CO7K 16 / 241 ( 2013 .01 ) ; A61K 39 / 3955 ANTIBODIES ( 2013 .01 ) ; A61K 31 /4706 ( 2013 .01 ) ; A61K 31 / 165 ( 2013 .01 ) ; CO7K 2317 /21 (2013 . 01 ) ; (71 ) Applicant: Eli D Ehrenpreis , Skokie , IL (US ) CO7K 2317/ 24 ( 2013. 01 ) ; A61K 2039/ 505 ( 2013 .01 ) (72 ) Inventor : Eli D Ehrenpreis, Skokie , IL (US ) (57 ) ABSTRACT Disclosed are methods for enhancing the efficacy of mono (21 ) Appl. No. : 15 /605 ,212 clonal antibody therapy , which entails co - administering a therapeutic monoclonal antibody , or a functional fragment (22 ) Filed : May 25 , 2017 thereof, and an effective amount of colchicine or hydroxy chloroquine , or a combination thereof, to a patient in need Related U . S . Application Data thereof . Also disclosed are methods of prolonging or increasing the time a monoclonal antibody remains in the (63 ) Continuation - in - part of application No . 14 / 947 , 193 , circulation of a patient, which entails co - administering a filed on Nov. 20 , 2015 . therapeutic monoclonal antibody , or a functional fragment ( 60 ) Provisional application No . 62/ 082, 682 , filed on Nov . of the monoclonal antibody , and an effective amount of 21 , 2014 . colchicine or hydroxychloroquine , or a combination thereof, to a patient in need thereof, wherein the time themonoclonal antibody remains in the circulation ( e . g . , blood serum ) of the Publication Classification patient is increased relative to the same regimen of admin (51 ) Int .