Timmerman Et Al-2020-Urelumab
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Predictive QSAR Tools to Aid in Early Process Development of Monoclonal Antibodies
Predictive QSAR tools to aid in early process development of monoclonal antibodies John Micael Andreas Karlberg Published work submitted to Newcastle University for the degree of Doctor of Philosophy in the School of Engineering November 2019 Abstract Monoclonal antibodies (mAbs) have become one of the fastest growing markets for diagnostic and therapeutic treatments over the last 30 years with a global sales revenue around $89 billion reported in 2017. A popular framework widely used in pharmaceutical industries for designing manufacturing processes for mAbs is Quality by Design (QbD) due to providing a structured and systematic approach in investigation and screening process parameters that might influence the product quality. However, due to the large number of product quality attributes (CQAs) and process parameters that exist in an mAb process platform, extensive investigation is needed to characterise their impact on the product quality which makes the process development costly and time consuming. There is thus an urgent need for methods and tools that can be used for early risk-based selection of critical product properties and process factors to reduce the number of potential factors that have to be investigated, thereby aiding in speeding up the process development and reduce costs. In this study, a framework for predictive model development based on Quantitative Structure- Activity Relationship (QSAR) modelling was developed to link structural features and properties of mAbs to Hydrophobic Interaction Chromatography (HIC) retention times and expressed mAb yield from HEK cells. Model development was based on a structured approach for incremental model refinement and evaluation that aided in increasing model performance until becoming acceptable in accordance to the OECD guidelines for QSAR models. -

RT+IO Therapy in NSCLC Draft 4 Clinical Cancer Research 1
Author Manuscript Published OnlineFirst on June 26, 2018; DOI: 10.1158/1078-0432.CCR-17-3620 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. RT+IO Therapy in NSCLC Draft 4 Clinical Cancer Research Title: The Integration of Radiotherapy With Immunotherapy for the Treatment of Non-Small Cell Lung Cancer Running title: Radiotherapy and Immunotherapy in Non-Small Cell Lung Cancer Eric C. Ko1, David Raben2, Silvia C. Formenti1 1Department of Radiation Oncology, Weill Cornell Medicine, New York, New York 2Department of Radiation Oncology, University of Colorado Anschutz Medical Campus, Aurora, Colorado Corresponding Author: Silvia C. Formenti, New York-Presbyterian/Weill Cornell Medicine, 525 East 68th Street, N-046, Box 169, New York, NY 10065-4885; Phone: 212-746-3608; Fax: 212-746-8850; E-mail: [email protected]. Confirmed Target Journal: Clinical Cancer Research Journal Specs (Review Article): Word Count (limit 3750 words): 4090 Abstract Word Count (unstructured, limit ≤250 words): 209 Number of References (≤75): 79 Number of Figures/Tables (5): 1 table, 3 figures 1 Downloaded from clincancerres.aacrjournals.org on September 24, 2021. © 2018 American Association for Cancer Research. Author Manuscript Published OnlineFirst on June 26, 2018; DOI: 10.1158/1078-0432.CCR-17-3620 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. RT+IO Therapy in NSCLC Draft 4 Clinical Cancer Research Abstract Five-year survival rates for non-small cell lung cancer (NSCLC) range from 14% to 49% for stage I to stage IIIA disease, and are <5% for stage IIIB/IV disease. -

ESCMID Online Lecture Library @ by Author
The diverse monoclonal antibodies in immunology and medical oncology: relevance for infectious diseases Dra. Isabel Ruiz Camps ESCMIDHospital Online Universitari Lecture Vall d’Hebron Library @ by authorBarcelona Disclosure • Astellas • Gilead Sciences • MSD • Novartis • Pfizer ESCMID Online Lecture Library @ by author A huge topic for such a ESCMIDshort Online time Lecture Library @ by author natalizumab antiTNF antiCD20: rituximab, obinutuzumab, ofatumumab gemtuzumab (antiCD33) alemtuzumab (antiCD52) daratumumab (antiCD38) Inotuzumab (antiCD22) Brentuximab (CD30) Seculinumab (anti IL-17) Tocilizumab (antiIL6) PI3K inhibitors PARP inh: olaparib, Guselkumab (anti IL12/23) rucaparib (copanlisib, more ... Roxulitinib, tofacitinib (JAK inh) Urelumab (CD137 R) Immunotherapy: ipilimumab TK inhibitors : Imatinib, dasatinib, Belimumab (antiBAFF) (CTLA-4), tremelimumab (CTLA-4), masitinib, bosutinib, nilotinib, fostamatinib nivolimumab (PD1/PDL1), (spleen), ibrutinib (BTK), alisertib (ATK), Pembrolizumab (PD1), more...... afatinib Cabozantinib: MET, RET, VEGFR2 HER2/neu:ESCMID trastuzumab, lapatinib Online VEGFR: bevacizumabLecture, LibrarymTOR: temsirolimus sorafenib, sunitinib EGFR: cetuximab, panitumumab, MAPK inh: dabrafenib, vemurafenib erlotinib, gefitinib Selinexor (XPO1 antagonist) @ by author Trametinib (MEK inh) Index • Background • Biological therapies for immunological diseases and risk of infection • Biological therapies for cancer – Target pathways – Risk of infection • Prevention ESCMID Online Lecture Library @ by author What are -

Immune Checkpoint Inhibition in DLBCL Immunotherapy: “The Cure Is Inside Us”
Mariano Provencio Servicio de Oncología Médica Hospital Universitario Puerta de Hierro Immune checkpoint inhibition in DLBCL Immunotherapy: “The Cure is Inside Us” § Our immune system prevents or limit infections by foreign antigens expressed in microorganisms (bacteria, viruses, etc.) § Our immune system can also recognize and destroy cancer cells….. • However, cancer cells have developed “escape mechanisms” to avoid their destruction by immune cells…“put the brakes on” • Immuno-Oncology: Find ways of “unleashing” the power of our body’s immune system to treat or prevent cancer…T-lymphocytes (T- cells) Detectives Dendritic cells Killer –T cells Microenvironment antigen (flags) Detectives Dendritic cells Killer –T cells Tumor infiltranting T cell recognizable ags Cor e Algorithms Margin Neo-antigens antigen (flags) 5 6 Scott DW et al. Nature Rev 2014 Strategy approach § Effective immune response: barriers § microenviroment § Activate anti-tumor immune response § inhibitory receptors: blocking antibodies § Nivo, Pembro, Ipi,…(anti PD 1) (CTLA4) § combining 2 checkpoint inhibitors § combining with chemotherapy § activate receptors: agonist § Urelumab § Utumilumab § Varlilumab Strategy approach § Effective immune response: barriers § microenviroment Inactivated effector T cell angiogenesis metabolism Strategy approach PDL-1 Effective immune CTLA-4 response: barriers Lymphoma PDL-1 PD-1 PDL-1 TIM-3 PDL-1 PDL-1 mTOR LAG-3 OXPHOS MHC1 Aerobic glycolysis Interferon gamma EB virus T cell activation antigen presenting cells Strategy approach § -
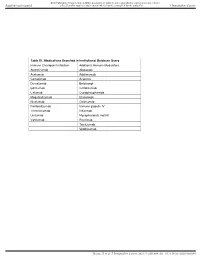
Table S1. Medications Searched in Institutional Database Query Immune Checkpoint Inhibitors Additional Immune Modulators Atezol
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) J Immunother Cancer Table S1. Medications Searched in Institutional Database Query Immune Checkpoint Inhibitors Additional Immune Modulators Atezolizumab Abatacept Avelumab Adalimumab Cemiplimab Anakinra Durvalumab Belatacept Ipilimumab Certolizumab Lirilumab Cyclophosphamide Mogamulizumab Etanercept Nivolumab Golimumab Pembrolizumab Immune globulin IV Tremelimumab Infliximab Urelumab Mycophenolate mofetil Varlilumab Rituximab Tocilizumab Vedolizumab Beattie J, et al. J Immunother Cancer 2021; 9:e001884. doi: 10.1136/jitc-2020-001884 BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) J Immunother Cancer Table S2. Toxicity of additional immune modulators Treatment detail Toxicity Patient 1 day 18,32 Infliximab dosed day 59 P.aeruginosa, S.marcescens pneumonia; died Patient 2 day 9 Infliximab dosed day 44 Febrile neutropenia; P. aeruginosa SBP and day 26 Mycophenolate initiated sepsis; C. albicans fungemia; treated and discharged Patient 8 day 4,11 Infliximab dosed day 14 Disseminated HSV-1; died Patient 26 day 79-128 Infliximab dosed (x7) day 130 E. faecalis, P. aeruginosa bacteremia; died; day 81, 97 Cyclophosphamide dosed Fungal pneumonia on autopsy Beattie J, et al. J Immunother Cancer 2021; 9:e001884. doi: 10.1136/jitc-2020-001884 BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) J Immunother Cancer Table S3. -

Immunfarmakológia Immunfarmakológia
Gergely: Immunfarmakológia Immunfarmakológia Prof Gergely Péter Az immunpatológiai betegségek döntő többsége gyulladásos, és ennek következtében általában szövetpusztulással járó betegség, melyben – jelenleg – a terápia alapvetően a gyulladás csökkentésére és/vagy megszűntetésére irányul. Vannak kizárólag gyulladásgátló gyógyszereink és vannak olyanok, amelyek az immunreakció(k) bénításával (=immunszuppresszió révén) vagy emellett vezetnek a gyulladás mérsékléséhez. Mind szerkezetileg, mind hatástanilag igen sokféle csoportba oszthatók, az alábbi felosztás elsősorban didaktikus célokat szolgál. 1. Nem-szteroid gyulladásgátlók (‘nonsteroidal antiinflammatory drugs’ NSAID) 2. Kortikoszteroidok 3. Allergia-elleni szerek (antiallergikumok) 4. Sejtoszlás-gátlók (citosztatikumok) 5. Nem citosztatikus hatású immunszuppresszív szerek 6. Egyéb gyulladásgátlók és immunmoduláns szerek 7. Biológiai terápia 1. Nem-szteroid gyulladásgátlók (NSAID) Ezeket a vegyületeket, melyek őse a szalicilsav (jelenleg, mint acetilszalicilsav ‘aszpirin’ használatos), igen kiterjedten alkalmazzák a reumatológiában, az onkológiában és az orvostudomány szinte minden ágában, ahol fájdalom- és lázcsillapításra van szükség. Egyes felmérések szerint a betegek egy ötöde szed valamilyen NSAID készítményt. Szerkezetük alapján a készítményeket több csoportba sorolhatjuk: szalicilátok (pl. acetilszalicilsav) pyrazolidinek (pl. fenilbutazon) ecetsav származékok (pl. indometacin) fenoxiecetsav származékok (pl. diclofenac, aceclofenac)) oxicamok (pl. piroxicam, meloxicam) propionsav -

The Two Tontti Tudiul Lui Hi Ha Unit
THETWO TONTTI USTUDIUL 20170267753A1 LUI HI HA UNIT ( 19) United States (12 ) Patent Application Publication (10 ) Pub. No. : US 2017 /0267753 A1 Ehrenpreis (43 ) Pub . Date : Sep . 21 , 2017 ( 54 ) COMBINATION THERAPY FOR (52 ) U .S . CI. CO - ADMINISTRATION OF MONOCLONAL CPC .. .. CO7K 16 / 241 ( 2013 .01 ) ; A61K 39 / 3955 ANTIBODIES ( 2013 .01 ) ; A61K 31 /4706 ( 2013 .01 ) ; A61K 31 / 165 ( 2013 .01 ) ; CO7K 2317 /21 (2013 . 01 ) ; (71 ) Applicant: Eli D Ehrenpreis , Skokie , IL (US ) CO7K 2317/ 24 ( 2013. 01 ) ; A61K 2039/ 505 ( 2013 .01 ) (72 ) Inventor : Eli D Ehrenpreis, Skokie , IL (US ) (57 ) ABSTRACT Disclosed are methods for enhancing the efficacy of mono (21 ) Appl. No. : 15 /605 ,212 clonal antibody therapy , which entails co - administering a therapeutic monoclonal antibody , or a functional fragment (22 ) Filed : May 25 , 2017 thereof, and an effective amount of colchicine or hydroxy chloroquine , or a combination thereof, to a patient in need Related U . S . Application Data thereof . Also disclosed are methods of prolonging or increasing the time a monoclonal antibody remains in the (63 ) Continuation - in - part of application No . 14 / 947 , 193 , circulation of a patient, which entails co - administering a filed on Nov. 20 , 2015 . therapeutic monoclonal antibody , or a functional fragment ( 60 ) Provisional application No . 62/ 082, 682 , filed on Nov . of the monoclonal antibody , and an effective amount of 21 , 2014 . colchicine or hydroxychloroquine , or a combination thereof, to a patient in need thereof, wherein the time themonoclonal antibody remains in the circulation ( e . g . , blood serum ) of the Publication Classification patient is increased relative to the same regimen of admin (51 ) Int . -

A Review of Current Biologics Targeting OX40, 4-1BB, CD27, and GITR
· TUMOR NECROSIS RECEPTOR AGONISTS · TNFR Agonists: A Review of Current Biologics Targeting OX40, 4-1BB, CD27, and GITR Elizabeth R. Sturgill, PhD, and William L. Redmond, PhD Abstract these are commonly known as T-cell priming. However, The tumor microenvironment is often immunosup- in addition to the initial priming event, further signaling pressive and lacks the appropriate signals necessary is necessary to drive T-cell differentiation and potentiate for stimulating effective anti-tumor T-cell responses. the development of effector and memory cell subsets; Therefore, considerable effort in the field of immu- these events enable robust tumor killing activity, in vivo. notherapy has been dedicated to developing new The tumor necrosis factor receptors (TNFRs), including biologics that target tumor necrosis factor receptors glucocorticoid-induced TNFR (GITR; CD357), CD27, (TNFRs). Signaling through TNFRs elicits a cascade of OX40 (CD134), and 4-1BB (CD137), are a family of proteins events critical for overcoming immune suppression, responsible for transducing these additional costimula- including T-cell activation, differentiation, and the devel- tory signals. In order to harness this important signaling opment of long-lived memory cells. In this review, we cascade, agonist mAbs and specific ligand complexes have discuss the biology of TNFRs, the current preclinical been developed that can engage with these TNFRs and ac- studies and clinical trials targeting these receptors, tivate downstream events. In this review, we will discuss the and how they may be combined to synergize with biology of TNFRs, the current preclinical and clinical trials other therapies to improve patient outcomes. targeting these receptors, and potential synergy with other AJHO. -

WO 2018/027204 Al 08 February 2018 (08.02.2018) W !P O PCT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2018/027204 Al 08 February 2018 (08.02.2018) W !P O PCT (51) International Patent Classification: only): F. HOFFMANN-LA ROCHE AG [CH/CH]; Gren- C07K 16/28 (2006.01) A61K 39/00 (2006.01) zacherstrasse 124, 4070 Basel (CH). (21) International Application Number: (72) Inventor; and PCT/US20 17/045642 (71) Applicant: HARRIS, Seth [US/US]; c/o Genentech, Inc., 1 DNA Way, South San Francisco, California 94080 (US). (22) International Filing Date: 04 August 2017 (04.08.2017) (72) Inventors: LAZAR, Greg; c/o Genentech, Inc., 1 DNA Way, South San Francisco, California 94080 (US). YANG, (25) Filing Language: English Yanli; c/o Genentech, Inc., 1 DNA Way, South San Fran (26) Publication Language: English cisco, California 94080 (US). CHRISTENSEN, Erin H.; c/ o Genentech, Inc., 1 DNA Way, South San Francisco, Cali (30) Priority Data: fornia 94080 (US). HANG, Julie; 6606 Wisteria Way, San 62/371,671 05 August 2016 (05.08.2016) US Jose, California 95 129 (US). KIM, Jeong; c/o Genentech, (71) Applicant (for all designated States except AL, AT, BA, BE, Inc., 1 DNA Way, South San Francisco, California 94080 BG, CH, CN, CY, CZ, DE, DK, EE, ES, FI, FR, GB, GR, (US). HR, HU, IE, IN, IS, IT, LT, LU, LV, MC, MK, MT, NL, (74) Agent: JONES, Kevin et al; Morrison & Foerster LLP, NO, PL, PT RO, RS, SE, SI, SK, SM, TR): GENENTECH, 425 Market Street, San Francisco, California 94105-2482 INC. -

WO 2016/176089 Al 3 November 2016 (03.11.2016) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/176089 Al 3 November 2016 (03.11.2016) P O P C T (51) International Patent Classification: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, A01N 43/00 (2006.01) A61K 31/33 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (21) International Application Number: KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, PCT/US2016/028383 MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (22) International Filing Date: PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, 20 April 2016 (20.04.2016) SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (25) Filing Language: English (84) Designated States (unless otherwise indicated, for every (26) Publication Language: English kind of regional protection available): ARIPO (BW, GH, (30) Priority Data: GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, 62/154,426 29 April 2015 (29.04.2015) US TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, (71) Applicant: KARDIATONOS, INC. [US/US]; 4909 DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, Lapeer Road, Metamora, Michigan 48455 (US). -

Ep 3178848 A1
(19) TZZ¥__T (11) EP 3 178 848 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 14.06.2017 Bulletin 2017/24 C07K 16/28 (2006.01) A61K 39/395 (2006.01) C07K 16/30 (2006.01) (21) Application number: 15198715.3 (22) Date of filing: 09.12.2015 (84) Designated Contracting States: (72) Inventor: The designation of the inventor has not AL AT BE BG CH CY CZ DE DK EE ES FI FR GB yet been filed GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR (74) Representative: Cueni, Leah Noëmi et al Designated Extension States: F. Hoffmann-La Roche AG BA ME Patent Department Designated Validation States: Grenzacherstrasse 124 MA MD 4070 Basel (CH) (71) Applicant: F. Hoffmann-La Roche AG 4070 Basel (CH) (54) TYPE II ANTI-CD20 ANTIBODY FOR REDUCING FORMATION OF ANTI-DRUG ANTIBODIES (57) The present invention relates to methods of treating a disease, and methods for reduction of the formation of anti-drug antibodies (ADAs) in response to the administration of a therapeutic agent comprising administration of a Type II anti-CD20 antibody, e.g. obinutuzumab, to the subject prior to administration of the therapeutic agent. EP 3 178 848 A1 Printed by Jouve, 75001 PARIS (FR) EP 3 178 848 A1 Description Field of the Invention 5 [0001] The present invention relates to methods of treating a disease, and methods for reduction of the formation of anti-drug antibodies (ADAs) in response to the administration of a therapeutic agent. -

(INN) for Biological and Biotechnological Substances
INN Working Document 05.179 Update 2013 International Nonproprietary Names (INN) for biological and biotechnological substances (a review) INN Working Document 05.179 Distr.: GENERAL ENGLISH ONLY 2013 International Nonproprietary Names (INN) for biological and biotechnological substances (a review) International Nonproprietary Names (INN) Programme Technologies Standards and Norms (TSN) Regulation of Medicines and other Health Technologies (RHT) Essential Medicines and Health Products (EMP) International Nonproprietary Names (INN) for biological and biotechnological substances (a review) © World Health Organization 2013 All rights reserved. Publications of the World Health Organization are available on the WHO web site (www.who.int ) or can be purchased from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected] ). Requests for permission to reproduce or translate WHO publications – whether for sale or for non-commercial distribution – should be addressed to WHO Press through the WHO web site (http://www.who.int/about/licensing/copyright_form/en/index.html ). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned.