A Review of Current Biologics Targeting OX40, 4-1BB, CD27, and GITR
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Predictive QSAR Tools to Aid in Early Process Development of Monoclonal Antibodies
Predictive QSAR tools to aid in early process development of monoclonal antibodies John Micael Andreas Karlberg Published work submitted to Newcastle University for the degree of Doctor of Philosophy in the School of Engineering November 2019 Abstract Monoclonal antibodies (mAbs) have become one of the fastest growing markets for diagnostic and therapeutic treatments over the last 30 years with a global sales revenue around $89 billion reported in 2017. A popular framework widely used in pharmaceutical industries for designing manufacturing processes for mAbs is Quality by Design (QbD) due to providing a structured and systematic approach in investigation and screening process parameters that might influence the product quality. However, due to the large number of product quality attributes (CQAs) and process parameters that exist in an mAb process platform, extensive investigation is needed to characterise their impact on the product quality which makes the process development costly and time consuming. There is thus an urgent need for methods and tools that can be used for early risk-based selection of critical product properties and process factors to reduce the number of potential factors that have to be investigated, thereby aiding in speeding up the process development and reduce costs. In this study, a framework for predictive model development based on Quantitative Structure- Activity Relationship (QSAR) modelling was developed to link structural features and properties of mAbs to Hydrophobic Interaction Chromatography (HIC) retention times and expressed mAb yield from HEK cells. Model development was based on a structured approach for incremental model refinement and evaluation that aided in increasing model performance until becoming acceptable in accordance to the OECD guidelines for QSAR models. -

RT+IO Therapy in NSCLC Draft 4 Clinical Cancer Research 1
Author Manuscript Published OnlineFirst on June 26, 2018; DOI: 10.1158/1078-0432.CCR-17-3620 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. RT+IO Therapy in NSCLC Draft 4 Clinical Cancer Research Title: The Integration of Radiotherapy With Immunotherapy for the Treatment of Non-Small Cell Lung Cancer Running title: Radiotherapy and Immunotherapy in Non-Small Cell Lung Cancer Eric C. Ko1, David Raben2, Silvia C. Formenti1 1Department of Radiation Oncology, Weill Cornell Medicine, New York, New York 2Department of Radiation Oncology, University of Colorado Anschutz Medical Campus, Aurora, Colorado Corresponding Author: Silvia C. Formenti, New York-Presbyterian/Weill Cornell Medicine, 525 East 68th Street, N-046, Box 169, New York, NY 10065-4885; Phone: 212-746-3608; Fax: 212-746-8850; E-mail: [email protected]. Confirmed Target Journal: Clinical Cancer Research Journal Specs (Review Article): Word Count (limit 3750 words): 4090 Abstract Word Count (unstructured, limit ≤250 words): 209 Number of References (≤75): 79 Number of Figures/Tables (5): 1 table, 3 figures 1 Downloaded from clincancerres.aacrjournals.org on September 24, 2021. © 2018 American Association for Cancer Research. Author Manuscript Published OnlineFirst on June 26, 2018; DOI: 10.1158/1078-0432.CCR-17-3620 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. RT+IO Therapy in NSCLC Draft 4 Clinical Cancer Research Abstract Five-year survival rates for non-small cell lung cancer (NSCLC) range from 14% to 49% for stage I to stage IIIA disease, and are <5% for stage IIIB/IV disease. -

Celldex Therapeutics Presents Phase 1 Study of Varlilumab and Opdivo® at 2017 ASCO Annual Meeting
June 5, 2017 Celldex Therapeutics Presents Phase 1 Study of Varlilumab and Opdivo® at 2017 ASCO Annual Meeting Early signs of clinical activity without increased toxicity observed HAMPTON, N.J., June 05, 2017 (GLOBE NEWSWIRE) -- Celldex Therapeutics, Inc. (Nasdaq:CLDX) announced today data from the Phase 1 portion of a Phase 1/2 dose escalation and cohort expansion study examining the combination of varlilumab, Celldex's CD27 targeting investigational immune-activating antibody, and Bristol-Myers Squibb's anti-PD-1 immunotherapy Opdivo® (nivolumab). Rachel E. Sanborn, M.D., Co-director of the Providence Thoracic Oncology Program; and Phase I Clinical Trials Program, at the Earle A. Chiles Research Institute, Providence Cancer Center, in Portland, Ore. presented results from the study in an oral presentation entitled, "Clinical Results with Combination of Anti-CD27 Agonist Antibody, Varlilumab, with Anti-PD1 Antibody Nivolumab in Advanced Cancer Patients" at the 2017 American Society of Clinical Oncology (ASCO) Annual Meeting in Chicago. The primary objective of the Phase 1 portion (n=36) of the study was to evaluate the safety and tolerability of the combination. The Phase 2 portion of the study is expected to complete enrollment in early 2018. "Combining PD-1 inhibition with a potent T cell activating agent provides the opportunity to broaden the number of patients that benefit from checkpoint blockade," said Dr. Sanborn. "While early, we have evidence that this combination does not add toxicity, can turn some ‘immune-cold' tumors hot, and may have clinical benefit, including in some patients who are not likely to respond to monotherapy. Further elucidating the role of intermittent versus chronic T cell activation through the comparison of alternate varlilumab dosing regimens is an essential component of the ongoing Phase 2 study and could be important in optimizing the potential of this combination." Key Highlights • The majority of patients enrolled in the study had PD-L1 negative tumor at baseline and presented with Stage IV, heavily- pretreated disease. -

ESCMID Online Lecture Library @ by Author
The diverse monoclonal antibodies in immunology and medical oncology: relevance for infectious diseases Dra. Isabel Ruiz Camps ESCMIDHospital Online Universitari Lecture Vall d’Hebron Library @ by authorBarcelona Disclosure • Astellas • Gilead Sciences • MSD • Novartis • Pfizer ESCMID Online Lecture Library @ by author A huge topic for such a ESCMIDshort Online time Lecture Library @ by author natalizumab antiTNF antiCD20: rituximab, obinutuzumab, ofatumumab gemtuzumab (antiCD33) alemtuzumab (antiCD52) daratumumab (antiCD38) Inotuzumab (antiCD22) Brentuximab (CD30) Seculinumab (anti IL-17) Tocilizumab (antiIL6) PI3K inhibitors PARP inh: olaparib, Guselkumab (anti IL12/23) rucaparib (copanlisib, more ... Roxulitinib, tofacitinib (JAK inh) Urelumab (CD137 R) Immunotherapy: ipilimumab TK inhibitors : Imatinib, dasatinib, Belimumab (antiBAFF) (CTLA-4), tremelimumab (CTLA-4), masitinib, bosutinib, nilotinib, fostamatinib nivolimumab (PD1/PDL1), (spleen), ibrutinib (BTK), alisertib (ATK), Pembrolizumab (PD1), more...... afatinib Cabozantinib: MET, RET, VEGFR2 HER2/neu:ESCMID trastuzumab, lapatinib Online VEGFR: bevacizumabLecture, LibrarymTOR: temsirolimus sorafenib, sunitinib EGFR: cetuximab, panitumumab, MAPK inh: dabrafenib, vemurafenib erlotinib, gefitinib Selinexor (XPO1 antagonist) @ by author Trametinib (MEK inh) Index • Background • Biological therapies for immunological diseases and risk of infection • Biological therapies for cancer – Target pathways – Risk of infection • Prevention ESCMID Online Lecture Library @ by author What are -

Classification Decisions Taken by the Harmonized System Committee from the 47Th to 60Th Sessions (2011
CLASSIFICATION DECISIONS TAKEN BY THE HARMONIZED SYSTEM COMMITTEE FROM THE 47TH TO 60TH SESSIONS (2011 - 2018) WORLD CUSTOMS ORGANIZATION Rue du Marché 30 B-1210 Brussels Belgium November 2011 Copyright © 2011 World Customs Organization. All rights reserved. Requests and inquiries concerning translation, reproduction and adaptation rights should be addressed to [email protected]. D/2011/0448/25 The following list contains the classification decisions (other than those subject to a reservation) taken by the Harmonized System Committee ( 47th Session – March 2011) on specific products, together with their related Harmonized System code numbers and, in certain cases, the classification rationale. Advice Parties seeking to import or export merchandise covered by a decision are advised to verify the implementation of the decision by the importing or exporting country, as the case may be. HS codes Classification No Product description Classification considered rationale 1. Preparation, in the form of a powder, consisting of 92 % sugar, 6 % 2106.90 GRIs 1 and 6 black currant powder, anticaking agent, citric acid and black currant flavouring, put up for retail sale in 32-gram sachets, intended to be consumed as a beverage after mixing with hot water. 2. Vanutide cridificar (INN List 100). 3002.20 3. Certain INN products. Chapters 28, 29 (See “INN List 101” at the end of this publication.) and 30 4. Certain INN products. Chapters 13, 29 (See “INN List 102” at the end of this publication.) and 30 5. Certain INN products. Chapters 28, 29, (See “INN List 103” at the end of this publication.) 30, 35 and 39 6. Re-classification of INN products. -

Tanibirumab (CUI C3490677) Add to Cart
5/17/2018 NCI Metathesaurus Contains Exact Match Begins With Name Code Property Relationship Source ALL Advanced Search NCIm Version: 201706 Version 2.8 (using LexEVS 6.5) Home | NCIt Hierarchy | Sources | Help Suggest changes to this concept Tanibirumab (CUI C3490677) Add to Cart Table of Contents Terms & Properties Synonym Details Relationships By Source Terms & Properties Concept Unique Identifier (CUI): C3490677 NCI Thesaurus Code: C102877 (see NCI Thesaurus info) Semantic Type: Immunologic Factor Semantic Type: Amino Acid, Peptide, or Protein Semantic Type: Pharmacologic Substance NCIt Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor tyrosine kinase expressed by endothelial cells, while VEGF is overexpressed in many tumors and is correlated to tumor progression. PDQ Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor -

Timmerman Et Al-2020-Urelumab
Urelumab alone or in combination with rituximab in patients with relapsed or refractory B-cell lymphoma John Timmerman, Charles Herbaux, Vincent Ribrag, Andrew D. Zelenetz, Roch Houot, Sattva S. Neelapu, Theodore Logan, Izidore S. Lossos, Walter Urba, Gilles Salles, et al. To cite this version: John Timmerman, Charles Herbaux, Vincent Ribrag, Andrew D. Zelenetz, Roch Houot, et al.. Ure- lumab alone or in combination with rituximab in patients with relapsed or refractory B-cell lymphoma. American Journal of Hematology, Wiley, 2020, 95 (5), pp.510-520. 10.1002/ajh.25757. hal-02798009 HAL Id: hal-02798009 https://hal-univ-rennes1.archives-ouvertes.fr/hal-02798009 Submitted on 23 Jun 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License Received: 25 October 2019 Revised: 30 January 2020 Accepted: 5 February 2020 DOI: 10.1002/ajh.25757 RESEARCH ARTICLE Urelumab alone or in combination with rituximab in patients with relapsed or refractory B-cell lymphoma John Timmerman1 | Charles -

Immune Checkpoint Inhibition in DLBCL Immunotherapy: “The Cure Is Inside Us”
Mariano Provencio Servicio de Oncología Médica Hospital Universitario Puerta de Hierro Immune checkpoint inhibition in DLBCL Immunotherapy: “The Cure is Inside Us” § Our immune system prevents or limit infections by foreign antigens expressed in microorganisms (bacteria, viruses, etc.) § Our immune system can also recognize and destroy cancer cells….. • However, cancer cells have developed “escape mechanisms” to avoid their destruction by immune cells…“put the brakes on” • Immuno-Oncology: Find ways of “unleashing” the power of our body’s immune system to treat or prevent cancer…T-lymphocytes (T- cells) Detectives Dendritic cells Killer –T cells Microenvironment antigen (flags) Detectives Dendritic cells Killer –T cells Tumor infiltranting T cell recognizable ags Cor e Algorithms Margin Neo-antigens antigen (flags) 5 6 Scott DW et al. Nature Rev 2014 Strategy approach § Effective immune response: barriers § microenviroment § Activate anti-tumor immune response § inhibitory receptors: blocking antibodies § Nivo, Pembro, Ipi,…(anti PD 1) (CTLA4) § combining 2 checkpoint inhibitors § combining with chemotherapy § activate receptors: agonist § Urelumab § Utumilumab § Varlilumab Strategy approach § Effective immune response: barriers § microenviroment Inactivated effector T cell angiogenesis metabolism Strategy approach PDL-1 Effective immune CTLA-4 response: barriers Lymphoma PDL-1 PD-1 PDL-1 TIM-3 PDL-1 PDL-1 mTOR LAG-3 OXPHOS MHC1 Aerobic glycolysis Interferon gamma EB virus T cell activation antigen presenting cells Strategy approach § -
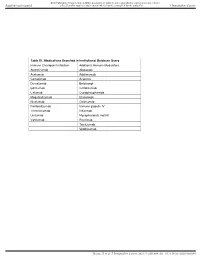
Table S1. Medications Searched in Institutional Database Query Immune Checkpoint Inhibitors Additional Immune Modulators Atezol
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) J Immunother Cancer Table S1. Medications Searched in Institutional Database Query Immune Checkpoint Inhibitors Additional Immune Modulators Atezolizumab Abatacept Avelumab Adalimumab Cemiplimab Anakinra Durvalumab Belatacept Ipilimumab Certolizumab Lirilumab Cyclophosphamide Mogamulizumab Etanercept Nivolumab Golimumab Pembrolizumab Immune globulin IV Tremelimumab Infliximab Urelumab Mycophenolate mofetil Varlilumab Rituximab Tocilizumab Vedolizumab Beattie J, et al. J Immunother Cancer 2021; 9:e001884. doi: 10.1136/jitc-2020-001884 BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) J Immunother Cancer Table S2. Toxicity of additional immune modulators Treatment detail Toxicity Patient 1 day 18,32 Infliximab dosed day 59 P.aeruginosa, S.marcescens pneumonia; died Patient 2 day 9 Infliximab dosed day 44 Febrile neutropenia; P. aeruginosa SBP and day 26 Mycophenolate initiated sepsis; C. albicans fungemia; treated and discharged Patient 8 day 4,11 Infliximab dosed day 14 Disseminated HSV-1; died Patient 26 day 79-128 Infliximab dosed (x7) day 130 E. faecalis, P. aeruginosa bacteremia; died; day 81, 97 Cyclophosphamide dosed Fungal pneumonia on autopsy Beattie J, et al. J Immunother Cancer 2021; 9:e001884. doi: 10.1136/jitc-2020-001884 BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) J Immunother Cancer Table S3. -

On the Horizon: Immuno-Oncology (I-O) Combinations
Immuno-Oncology (I-O) Combinations • Jeffrey A. Sosman, MD • Robert H. Lurie Comprehensive Cancer Center of Northwestern University The Cancer–Immunity Cycle Daniel Chen and Ira Mellman Immunity, Volume 39, Issue 1, 2013, 1 - 10 The Cancer–Immunity Cycle Daniel Chen and Ira Mellman Immunity, Volume 39, Issue 1, 2013, 1 - 10 Stimulatory and Inhibitory Factors in the Cancer-Immunity Cycle Each step of the Cancer-Immunity Cycle requires the coordination of numerous factors, both stimulatory and inhibitory in nature. Stimulatory factors shown in green promote immunity, ... Where will Improvements come from? • Combinations: – Based on Template: anti-PD-1/PD-L1 or with anti-PD- 1/anti-CTLA-4 • Block other co-inhibitory: LAG3, TIM3, KIR, VISTA • Activate co-stimulatory: 4-1BB, OX-40, GITR, CD27, ICOS • Block inhibitory molecules- IDOi, TGFbi, CSF1Ri, anti-IL-6 or anti- IL-10 • Effect trafficking- anti-VEGF, CCL5, CXCR4i • Vaccines- TVEC- oncolytic virus, Neoantigen, other cellular • Adoptive Cellular therapy- TIL, CAR-T cells, TCR T-cells Where will Improvements come from? • Combinations: – Based on Template: anti-PD-1/PD-L1 or with anti-PD- 1/anti-CTLA-4 • Signal Inhibition, BRAF directed (BRAFi+MEKi), MEKi, PI3K inhibition (PTEN effects) • Cytokines- IL-2, IFN a,b,g,, Directed cytokines (FAP-IL-2v or CEA-IL-2v) • Epigenetic modulation- gene expression and EVR expression • Microbiome modification- fecal transplants • Chemotherapy other cytotoxics • Localized Irradiation SBRT, SRS T cells in Tumors Express Multiple Immunoinhibitory Receptors -

Immunfarmakológia Immunfarmakológia
Gergely: Immunfarmakológia Immunfarmakológia Prof Gergely Péter Az immunpatológiai betegségek döntő többsége gyulladásos, és ennek következtében általában szövetpusztulással járó betegség, melyben – jelenleg – a terápia alapvetően a gyulladás csökkentésére és/vagy megszűntetésére irányul. Vannak kizárólag gyulladásgátló gyógyszereink és vannak olyanok, amelyek az immunreakció(k) bénításával (=immunszuppresszió révén) vagy emellett vezetnek a gyulladás mérsékléséhez. Mind szerkezetileg, mind hatástanilag igen sokféle csoportba oszthatók, az alábbi felosztás elsősorban didaktikus célokat szolgál. 1. Nem-szteroid gyulladásgátlók (‘nonsteroidal antiinflammatory drugs’ NSAID) 2. Kortikoszteroidok 3. Allergia-elleni szerek (antiallergikumok) 4. Sejtoszlás-gátlók (citosztatikumok) 5. Nem citosztatikus hatású immunszuppresszív szerek 6. Egyéb gyulladásgátlók és immunmoduláns szerek 7. Biológiai terápia 1. Nem-szteroid gyulladásgátlók (NSAID) Ezeket a vegyületeket, melyek őse a szalicilsav (jelenleg, mint acetilszalicilsav ‘aszpirin’ használatos), igen kiterjedten alkalmazzák a reumatológiában, az onkológiában és az orvostudomány szinte minden ágában, ahol fájdalom- és lázcsillapításra van szükség. Egyes felmérések szerint a betegek egy ötöde szed valamilyen NSAID készítményt. Szerkezetük alapján a készítményeket több csoportba sorolhatjuk: szalicilátok (pl. acetilszalicilsav) pyrazolidinek (pl. fenilbutazon) ecetsav származékok (pl. indometacin) fenoxiecetsav származékok (pl. diclofenac, aceclofenac)) oxicamok (pl. piroxicam, meloxicam) propionsav -

The Two Tontti Tudiul Lui Hi Ha Unit
THETWO TONTTI USTUDIUL 20170267753A1 LUI HI HA UNIT ( 19) United States (12 ) Patent Application Publication (10 ) Pub. No. : US 2017 /0267753 A1 Ehrenpreis (43 ) Pub . Date : Sep . 21 , 2017 ( 54 ) COMBINATION THERAPY FOR (52 ) U .S . CI. CO - ADMINISTRATION OF MONOCLONAL CPC .. .. CO7K 16 / 241 ( 2013 .01 ) ; A61K 39 / 3955 ANTIBODIES ( 2013 .01 ) ; A61K 31 /4706 ( 2013 .01 ) ; A61K 31 / 165 ( 2013 .01 ) ; CO7K 2317 /21 (2013 . 01 ) ; (71 ) Applicant: Eli D Ehrenpreis , Skokie , IL (US ) CO7K 2317/ 24 ( 2013. 01 ) ; A61K 2039/ 505 ( 2013 .01 ) (72 ) Inventor : Eli D Ehrenpreis, Skokie , IL (US ) (57 ) ABSTRACT Disclosed are methods for enhancing the efficacy of mono (21 ) Appl. No. : 15 /605 ,212 clonal antibody therapy , which entails co - administering a therapeutic monoclonal antibody , or a functional fragment (22 ) Filed : May 25 , 2017 thereof, and an effective amount of colchicine or hydroxy chloroquine , or a combination thereof, to a patient in need Related U . S . Application Data thereof . Also disclosed are methods of prolonging or increasing the time a monoclonal antibody remains in the (63 ) Continuation - in - part of application No . 14 / 947 , 193 , circulation of a patient, which entails co - administering a filed on Nov. 20 , 2015 . therapeutic monoclonal antibody , or a functional fragment ( 60 ) Provisional application No . 62/ 082, 682 , filed on Nov . of the monoclonal antibody , and an effective amount of 21 , 2014 . colchicine or hydroxychloroquine , or a combination thereof, to a patient in need thereof, wherein the time themonoclonal antibody remains in the circulation ( e . g . , blood serum ) of the Publication Classification patient is increased relative to the same regimen of admin (51 ) Int .