M. Sc II Semester (Biotechnology) Cells, Molecules and Developmental Biology Unit IV Types of Gametogenesis: Spermatogenesis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Preliminary Note on the Embryology of <Emphasis Type="Italic">Casuarina Equisetifolia </Emphasis>, Forst
A PRELIMINARY NOTE ON THE EMBRYOLOGY OF CASUARINA EQUISETIFOLIA, FORST BY B. G. L. SWAMs (Bangalore) Received June 27, 1944 (Communicated by Prof. L. S. S. Kumar, r.A.SC.) THE remarkable discovery of Chalazogamy in Casuarina by Treub in 1891 evoked very keen interest and initiated further studies of Casuarinaceae and Amentifera~ fi'om both morphological and anatomical points of view. Certain aspects of the megasporogenesis of Casuarhza stricta was subsequently studied by Frye in 1903 and Juel (1903) recorded his observations on the origin and development of the female archesporium in Casuarina quadrivalvis. In spite of these contributions our present knowledge regarding the develop- mental stages in the life-history are far from being satisfactory. An inves- tigation of several species of the genus has been taken up by the author and a few salient features in the life-history of Casuarina equiset~folia Forst have been embodied in this preliminary note. The archesporiurn of the microsporangium is subepidermal in origin and can be differentiated by rich cell contents and conspicuous nuclei. After the formation of the endothecium, wall layers and tapetum, the microspore mother cells undergo the usual stages of the reduction divisions and form quartets of microspores arranged tetrahedrally. The quartets round off and their nuclei undergo division into tube and generative cells The pollen grains at the shedding stage are binucleate. Each ovary contains two erect ovules which arise laterally from a basal placenta (Fig. 1). The ovules are bitegnmentary, the inner integument differentiating slightly earlier than the outer; these grow upwards and organise a micropyle. -

Ostrich Production Systems Part I: a Review
11111111111,- 1SSN 0254-6019 Ostrich production systems Food and Agriculture Organization of 111160mmi the United Natiorp str. ro ucti s ct1rns Part A review by Dr M.M. ,,hanawany International Consultant Part II Case studies by Dr John Dingle FAO Visiting Scientist Food and , Agriculture Organization of the ' United , Nations Ot,i1 The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. M-21 ISBN 92-5-104300-0 Reproduction of this publication for educational or other non-commercial purposes is authorized without any prior written permission from the copyright holders provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without written permission of the copyright holders. Applications for such permission, with a statement of the purpose and extent of the reproduction, should be addressed to the Director, Information Division, Food and Agriculture Organization of the United Nations, Viale dells Terme di Caracalla, 00100 Rome, Italy. C) FAO 1999 Contents PART I - PRODUCTION SYSTEMS INTRODUCTION Chapter 1 ORIGIN AND EVOLUTION OF THE OSTRICH 5 Classification of the ostrich in the animal kingdom 5 Geographical distribution of ratites 8 Ostrich subspecies 10 The North -

HAMMOUDI-Roukia.Pdf
UNIVERSITE KASDI MERBAH - OUARGLA Faculté des Sciences de la Nature et de la Vie Département des Sciences Biologiques Année : 2014-2015 N° d’enregistrement : /…./…./…./…./ THESE pour l’obtention du diplôme de Doctorat ès sciences en biologie Activités biologiques de quelques métabolites secondaires extraits de quelques plantes médicinales du Sahara méridional algérien présentée et soutenue publiquement par HAMMOUDI Roukia le 24/05/2015 devant le jury composé de : BISSATI-BOUAFIA Samia Professeur U.K.M. Ouargla Président HADJ MAHAMMED Mahfoud Professeur U.KM. Ouargla Rapporteur OULD EL HADJ Mohamed Didi Professeur U.KM. Ouargla Co –Rapporteur SANON Souleymane M.C.A. CNRFP Ouagadougou Examinateur CHERITI Abdelkrim Professeur U. Bechar Examinateur BOURAS Noureddine M.C.A. ENS Kouba Examinateur REMERCIEMENTS Tout d’abord, je remercie sincèrement Monsieur HADJ MAHAMMED M., Professeur à la faculté des Mathématiques et des Sciences de la Matière de l’Université KASDI MERBAH-Ouargla pour l’honneurs qu’il m’a fait en acceptant d’encadrer ce travail et pour la confiance qu’il m’a accordée et son accueil au laboratoire de Biogéochimie des Milieux Désertiques de l’université KASDI MERBAH, Ouargla. Mes vifs remerciements vont à Monsieur le Professeur OULD EL HADJ M.D., Professeur à la faculté des sciences de la nature et de la vie de l’Université KASDI MERBAH-Ouargla pour avoir co-dirigé ce travail, ainsi que pour ses conseils, ses encouragements et les nombreuses suggestions scientifiques qu’il m’a prodigué. Je remercie également Madame BISSATI-BOUAFIA S. Professeur et doyenne de notre faculté des sciences de la nature et de la vie de l’Université KASDI MERBAH-Ouargla, d’avoir accepté de présider le jury de cette thèse, et pour ses encouragements incessants. -

Live-Cell Imaging and Optical Manipulation of Arabidopsis Early Embryogenesis
Technology Live-Cell Imaging and Optical Manipulation of Arabidopsis Early Embryogenesis Graphical Abstract Authors Keita Gooh, Minako Ueda, Kana Aruga, ..., Hideyuki Arata, Tetsuya Higashiyama, Daisuke Kurihara Correspondence [email protected] In Brief Gooh et al. establish a live-embryo imaging system for Arabidopsis and generate a complete lineage tree from double fertilization in early embryogenesis. They provide a platform for real-time analysis of cell division dynamics and cell fate specification using optical manipulation and micro- engineering techniques such as laser irradiation of specific cells. Highlights d A live-embryo imaging system for Arabidopsis is established d A complete lineage tree from double fertilization to 64-cell embryo uses this system d Endosperm development is not required for cell patterning during early embryogenesis d Damage to an embryo initial cell induces rapid cell fate conversion in the suspensor Gooh et al., 2015, Developmental Cell 34, 242–251 July 27, 2015 ª2015 Elsevier Inc. http://dx.doi.org/10.1016/j.devcel.2015.06.008 Developmental Cell Technology Live-Cell Imaging and Optical Manipulation of Arabidopsis Early Embryogenesis Keita Gooh,1 Minako Ueda,1,2 Kana Aruga,1 Jongho Park,1,3,4 Hideyuki Arata,1,3 Tetsuya Higashiyama,1,2,3 and Daisuke Kurihara1,3,* 1Division of Biological Science, Graduate School of Science, Nagoya University, Furo-cho, Chikusa-ku, Nagoya, Aichi 464-8602, Japan 2Institute of Transformative Bio-Molecules (ITbM), Nagoya University, Furo-cho, Chikusa-ku, Nagoya, Aichi 464-8602, Japan 3Higashiyama Live-Holonics Project, ERATO, JST, Furo-cho, Chikusa-ku, Nagoya, Aichi 464-8602, Japan 4Present address: Precision and Intelligence Laboratory, Tokyo Institute of Technology, 4259 Nagatsuta-cho, Midori-ku, Yokohama, Kanagawa 226-8503, Japan *Correspondence: [email protected] http://dx.doi.org/10.1016/j.devcel.2015.06.008 SUMMARY to the embryo (Kawashima and Goldberg, 2010; Lau et al., 2012; Wendrich and Weijers, 2013). -

Protection of Washed and Pasteurized Shell Eggs Against Fungal Growth by Application of Natamycin-Containing Shellac Coating
Protection of Washed and Pasteurized Shell Eggs against Fungal Growth by Application of Natamycin-Containing Shellac Coating THESIS Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in the Graduate School of The Ohio State University By Yang Song Graduate Program in Food Science and Technology The Ohio State University 2016 Master's Examination Committee: Dr. Ahmed Yousef, Advisor Dr. Dennis R. Heldman Dr. Luis Rodriguez-Saona Copyrighted by Yang Song 2016 Abstract Mold contamination of commercial shell eggs can potentially cause significant economic loss to the egg industry during storage. Studies indicated that molds from varies sources can propagate on commercial eggs when storage condition is less ideal. The current egg processing procedures such as commercial washing and pasteurization can weaken the egg shell, which is the primary defense of egg content, and expose processed eggs to contaminations. Generally, processed eggs are coated with mineral oil to overcome this problem. However, oil application is not very effective when used to protect eggs against mold contamination during storage. The food grade anti-fungal agent natamycin can be used to improve egg defense against mold contamination; however, direct application on egg surface will cause it to lose activity rapidly. Therefore, incorporation of natamycin and a food-grade coating is necessary to extend its anti-fungal effectiveness. As a food-grade coating, shellac can retain egg quality better compare to other coating materials; moreover, it can also serve as a matrix for natamycin to treat egg surface. Research is needed to investigate whether natamycin can remain effective in shellac coating; determine the minimum inhibitory concentration (MIC) of natamycin in shellac coating against typical mold contaminants, and whether the natamycin-shellac coating is effective when used on commercial washed eggs and pasteurized eggs. -

Mind Your Eggs & Veggies
MIND YOUR EGGS & VEGGIES Nutrition for Cognitive Health Presented by Taylor C. Wallace, PhD, CFS, FACN And Chef Abbie Gellman, MS, RD, CDN Spread the Fruit and Veggie Love #haveaplant @fruits_veggies @fruitsandveggies @fruitsandveggies © 2020 Produce for Better Health Foundation 2 Our Purpose The Produce for Better Health Foundation (PBH), a 501(c)3, is the only national non-profit organization committed to helping people live happier, healthier lives by eating more fruits and vegetables in all their glorious forms every day. © 20202019 Produce for Better Health Foundation 3 Our Movement Research shows, rather than a prescriptive recommendation to eat a certain amount of fruits and vegetables each day, consumers (particularly Gen Z and Millennials) want actionable, realistic and FUN approaches that make eating fruits and vegetables easy, helping them feel confident, happy and healthy. That’s where PBH’s Have A Plant® movement comes in. It’s a way to tap into the emotional connection consumers have to the fruit and vegetable eating experience while inspiring long-term, sustainable behavior change. And it does so with a no-nonsense approach that’s simple, understandable, and, importantly for this audience, non-prescriptive. © 2020 Produce for Better Health Foundation 4 Moderator Wendy Reinhardt Kapsak, MS, RDN © 2020 Produce for Better Health Foundation 5 Content Series: Eggs: Veggies’ Reliable BFF © 2020 Produce for Better Health Foundation 6 Adding Eggs to Enhance the Benefits of Produce Taylor C. Wallace, PhD, CFS, FACN Think Healthy Group, Inc. George Mason University © 2020 Produce for Better Health Foundation 7 Disclosures • Think Healthy Group, Inc. • George Mason University • The Dr. -

2020 Front Cover
INDIANA STATE POULTRY ASSOCIATION PRESENTS: 2020 POULTRY INFOMATION BOOKLET THIS BOOKLET CONTAINS: Test Your Knowledge - Poultry Quiz (Inside Front Cover) Poultry Terms Quick Reference Guide (Pg. 1) Getting Started with a Home Poultry Flock (Pg. 2) Common Egg Shell Quality Problems (Pg. 5) Choosing a Chicken Breed: Eggs, Meat or Exhibition (Pg. 6) Stay Healthy When Working with Farm Animals (Pg. 9) Exhibition Poultry (Pg. 10) Proper Handling of Eggs: From Hen to Consumption (Pg. 12) Don’t Get Caught Without These Forms (Pg. 17) Avian Influenza Findings Emphasize the Need for Good Biosecurity (Pg. 18) Backyard Biosecurity Self Evaluation (Pg. 20) Indiana Test Twelve Flock Evaluation (Inside Back Cover) This Booklet is designed to be a quick resource for raising poultry. For additional information go to www.INpoultry.com INDIANA STATE POULTRY ASSOCIATION PURDUE UNIVERSITY, ANIMAL SCIENCES 270 SOUTH RUSSELL STREET • WEST LAFAYETTE, IN 47907 765-494-8517 • [email protected] • WWW.INPOULTRY.COM TEST YOUR KNOWLEDGE - POULTRY QUIZ THINK YOU KNOW POULTRY? ANSWER THESE TEN QUESTIONS FOR A CHANCE TO WIN A PRIZE. ALL ANSWERS CAN BE FOUND THROUGHOUT THIS BOOKLET. 1. If chicks move away from the heat source in their brooding area and appear to be drowsy, then the temperature is most likely too ________ . 2. What might be the cause of a misshapen egg? a. Immature shell gland b. Disease c. Stress d. Overcrowding e. All of the above 3. Chicken breeds with _______ ear lobes lay white eggs while chicken breeds with ______ ear lobes lay brown eggs. 4. Anyone can get sick from farm animals, but what groups of people are more likely to have a serious illness? a. -

An Introduction to Morphology of the Reproductive System and Anatomy of Hen's
J. Life Earth Sci., Vol. 8: 1-10, 2013 ISSN 1990-4827 http://banglajol.info.index.php/JLES © 2013, JLES, RU AN INTRODUCTION TO MORPHOLOGY OF THE REPRODUCTIVE SYSTEM AND ANATOMY OF HEN’S EGG Md. Anisur Rahman Department of Zoology, University of Rajshahi, Rajshahi-6205, Bangladesh e-mail: [email protected] Abstract: The present study was designed to investigate the morphology of reproductive system and egg anatomy of the domestic hen (Gallus domesticus L.). The system consists of oviduct and ovary. The oviduct consists of infundibulum, magnum, isthmus, uterus and vagina which are sole distributor for making nutrition enriched egg. The anatomy of egg revealed that there are calcareous eggshell, shell membranes, egg white, vitelline membrane, egg yolk, and germinal disc. The fertilized egg showed a concentric circle around the nucleus known as blastoderm that contained area pellucida and area opaca whereas an unfertilized egg showed nucleus as white spot (blastodisc). Primordial germ cells (PGCs) are progenitor cells of ova and spermatozoa and they originate from the epiblast of the central part of the area pellucida. The microscopic structure of eggshell rendered leathery cuticle, fibrous matrix and shell membranes. The egg protects itself by its own mechanism from being injured and provides a complete diet for the developing embryo. Key words: Ovary, oviduct, egg, blastodisc, eggshell mvivsk: eZ©gvb Aa¨qbwU gyiwMi (Gallus domesticus L.) cÖRbbZ‡š¿i A½ms¯’vb Ges wW‡gi A½e¨e‡”Q` AbymÜvb Kivi Rb¨ Av‡jLb Kiv n‡q‡Q| cÖRbbZš¿wU‡Z wW¤^bvjx -

In Vitro Fertilisation in Wheat (Triticum Aestivum L.)
Functional Plant Science and Biotechnology ©2007 Global Science Books In Vitro Fertilisation in Wheat (Triticum aestivum L.) Zsolt Pónya1* • Beáta Barnabás 2 • Mauro Cresti1 1 Università degli Studi di Siena Dipartimento di Scienze Ambientali “G. Sarfatti”, Via P. A. Mattioli, 4, 53100 Siena, Italy 2 Department of Cell Biology and Plant Physiology, Agricultural Research Institute of the Hungarian Academy of Sciences (ARI HAS), Brunszvik ùt 2., Martonvásár, 2462, Hungary Corresponding author : * [email protected] ABSTRACT Studying the early events of fertilisation and the first zygotic cleavage during zygotic embryogenesis in angiosperms requires meticulously designed, complex experimental approaches. In wheat, like in an overwhelming number of crop species, little is known about fertilisation and the early events that ensue concomitantly. Therefore, answering questions such as to what extent the wheat zygote is dependent on the endosperm for its normal development, what morhpogenetic stimuli come from the maternal tissues, what factors are necessary for the accomplishment of embryonic growth from the onset of zygote development, what intertwining pathways ensure egg activation triggered by fertilization is of paramount importance. Towards this goal a brief overview is provided as to the main achievements of in vitro fertilisation (IVF) in the best-described maize system. This paper summarizes experimental data obtained by the application of IVF and the microinjection method adopted in wheat to address some important issues such as the ultrastructural characterization of wheat egg cells isolated at different maturational stages; the competence of the wheat female gamete for transient expression of foreign genes; DNA dynamics during sperm-egg fusion and zygotic development; and cytoskeletal changes occurring during in situ egg cell development and in the course of fertilization. -

European Researcher. 2010
Russian Journal of Comparative Law, 2016, Vol. (10), Is. 4 Copyright © 2016 by Academic Publishing House Researcher Published in the Russian Federation Russian Journal of Comparative Law Has been issued since 2014. ISSN 2411-7994 E-ISSN 2413-7618 Vol. 10, Is. 4, pp. 140-153, 2016 DOI: 10.13187/rjcl.2016.10.140 http://ejournal41.com UDC 341.01 The Human Embryo in the Case-Law of the European Court of Human Rights Alla Tymofeyeva a , * a Charles University, the Czech Republic Abstract This paper presents an analysis of the case-law of the European Court of Human Rights regarding the status of a human embryo. At the beginning, the author makes an overview of the legal documents on the subject-matter produced by intergovernmental organisations on both universal and regional levels. The overview covers also a national legislation of Australia, China, Japan, United States and most of the European states. Further the paper elaborates on the decisions and judgments of the European Court adopted for the last fifty five years. The summary of this case-law is provided in a chronological order from the oldest to the newest one. Finally, the author comments on the practice of the European Court of Human Rights with regard to the issue of the human embryo legal position. The case-law in question certifies that human embryo cannot be considered “a thing”, but at the same time it is still not “a person” for the purposes of the Article 2 of the Convention. Keywords: European Court of Human Rights, European Convention on Human Rights, Court, Convention, human embryo, abortion, embryo research. -
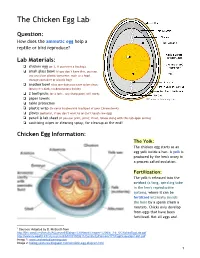
The Chicken Egg Lab 1
The Chicken Egg Lab1 Question: How does the amniotic egg help a reptile or bird reproduce? Lab Materials: ❏ chicken egg (or 2, if you want a backup) ❏ small glass bowl (if you don’t have this, you can use any clear plastic container, such as a food storage container or plastic bag) ❏ another bowl (this one does not have to be clear; ideally it’s dark, no decorations inside) ❏ 2 toothpicks (or a fork… any sharp point will work) ❏ paper towels ❏ table protection ❏ plastic wrap (to cover keyboard & trackpad of your Chromebook) ❏ gloves (optional, if you don’t want to or can’t touch raw egg) ❏ pencil & lab sheet (if you can print, print; if not, follow along with this tab open online) ❏ sanitizing wipes or cleaning spray, for cleanup at the end! Chicken Egg Information: The Yolk: The chicken egg starts as an egg yolk inside a hen. A yolk is produced by the hen's ovary in a process called ovulation. Fertilization: The yolk is released into the oviduct (a long, spiraling tube in the hen's reproductive system), where it can be fertilized internally (inside the hen) by a sperm (from a rooster). Chicks only develop from eggs that have been fertilized. Not all eggs are! 1 Sources: Adapted by D. McBeath from http://ljhs.sandi.net/faculty/AQuesnell/Biology%20Notes/Chapter%206/6_3-6_5/ChickenEggLab.pdf http://www.newpaltz.k12.ny.us/cms/lib/NY01000611/Centricity/Domain/171/EggDissectionLab1.pdf Image 1: www.enchantedlearning.com Image 2: biology-pictures.blogspot.com/amniotic-egg-diagram.html 1 The Egg White (albumin): The yolk continues down the oviduct (whether or not it is fertilized) and is covered with a membrane, structural fibers, and layers of albumin protein (the egg white). -

Review Yoshinori Mine and Jennifer Kovacs-Nolan
Journal of Poultry Science, .+ : +῎,3, ,**. ῌReview῍ Yoshinori Mine and Jennifer Kovacs-Nolan Department of Food Science, University of Guelph Guelph, Ontario N+G ,W+, Canada It is widely recognized that eggs are more than a source of dietary nutrients, and extensive studies identifying and characterizing the biologically active components of eggs have been carried out. Numerous biological activities have now been associated with egg components, including antibacterial and antiviral activity, immunomodulatory activity, and anti-cancer activity, indicating the importance of eggs and egg components in human health, and disease prevention and treatment. The potential of some of these biologically active components has already been realized, including egg white lysozyme and avidin, and yolk IgY and lecithin, which are currently produced on an industrial scale, and have been applied for the prevention and treatment of various medical conditions. The information presented here serves to demonstrate the significant potential of biologically active egg components, for medical, nutraceutical, and food- fortification applications. Key words : Hen eggs, bio-active components, nutraceuticals, health and disease, functional foods ῌῌῌῌῌῌῌῌῌῌῌῌῌῌῌῌῌῌῌῌῌῌῌ Yoshinori Mine +321 : M. Sc. (Food Science) Shinshu University, Japan +33- : Ph. D. (Biochemistry) Tokyo University of Agriculture and Technology, Japan Current position Associate Professor and Chair in Egg Material Sciences Department of Food Science University of Guelph. Area of research interests : Egg Material Sciences, Bio-active protein/peptides, Molecular biology of egg allergens. Jennifer Kovacs-Nolan ,**+ : M. Sc. (Food Science) University of Guelph , Ontario, Canada. Current position : Ph. D. student, Department of Food Science, University of Guelph, Ontario, Canada. Area of research interests : Food biochemistry/biotechnology.