Protection of Washed and Pasteurized Shell Eggs Against Fungal Growth by Application of Natamycin-Containing Shellac Coating
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Preliminary Note on the Embryology of <Emphasis Type="Italic">Casuarina Equisetifolia </Emphasis>, Forst
A PRELIMINARY NOTE ON THE EMBRYOLOGY OF CASUARINA EQUISETIFOLIA, FORST BY B. G. L. SWAMs (Bangalore) Received June 27, 1944 (Communicated by Prof. L. S. S. Kumar, r.A.SC.) THE remarkable discovery of Chalazogamy in Casuarina by Treub in 1891 evoked very keen interest and initiated further studies of Casuarinaceae and Amentifera~ fi'om both morphological and anatomical points of view. Certain aspects of the megasporogenesis of Casuarhza stricta was subsequently studied by Frye in 1903 and Juel (1903) recorded his observations on the origin and development of the female archesporium in Casuarina quadrivalvis. In spite of these contributions our present knowledge regarding the develop- mental stages in the life-history are far from being satisfactory. An inves- tigation of several species of the genus has been taken up by the author and a few salient features in the life-history of Casuarina equiset~folia Forst have been embodied in this preliminary note. The archesporiurn of the microsporangium is subepidermal in origin and can be differentiated by rich cell contents and conspicuous nuclei. After the formation of the endothecium, wall layers and tapetum, the microspore mother cells undergo the usual stages of the reduction divisions and form quartets of microspores arranged tetrahedrally. The quartets round off and their nuclei undergo division into tube and generative cells The pollen grains at the shedding stage are binucleate. Each ovary contains two erect ovules which arise laterally from a basal placenta (Fig. 1). The ovules are bitegnmentary, the inner integument differentiating slightly earlier than the outer; these grow upwards and organise a micropyle. -

COMMON QUESTIONS ABOUT EGGS by Jacquie Jacob and Tony Pescatore
PUB NUMBER (if applicable) COMMON QUESTIONS ABOUT EGGS By Jacquie Jacob and Tony Pescatore Question: Since eggs contain cholesterol, should I avoid eating eggs? No. Eggs are an excellent source of easy to digest, high quality protein that can play an important role in the diet of most Americans. It is true that high blood cholesterol levels have been shown to aggravate heart problems, but research has also shown that the presence or absence of eggs in the diet of most Americans is not likely to in- crease blood cholesterol levels. Only a small minority of people cannot regulate blood cholesterol levels. These people need to avoid high-cholesterol foods. One large egg contains about 213 milligrams (mg) of cholesterol—all of which is found in the yolk. The American Medical Association (AMA) recommends that, as long as you are healthy, you should limit your dietary cholesterol intake to less than 300 mg a day. If you have cardiovascular disease, diabetes or high LDL (or "bad") cholesterol, you should limit your dietary cholesterol intake to less than 200 mg a day. Therefore, if you eat an egg on a given day, it's important to limit or avoid other sources of cholesterol for the rest of that day. If you like eggs but do not want the cholesterol it contains, use egg whites which do not contain cholesterol. You can also use cholesterol-free egg substitutes, which are made with egg whites. If you want to reduce cholesterol in a recipe that calls for eggs, use two egg whites or 1/4 cup cholesterol-free egg substitute in place of one whole egg. -

Safest Choice™ Pasteurized Shell Eggs
Safest Choice™ Pasteurized Shell Eggs Cage Free also available Safest Choice™ Pasteurized Shell Eggs Safeguard your customers…and your reputation! Product Specs ™ Safest Choice Pasteurized Shell Eggs eliminate the risk of Storage Temp 33-40°F Salmonella from your egg dishes. The all-natural, patented pasteurization process destroys Salmonella, as well as avian Pallet Tie-Layer 12x5=60 Cases per pallet flu viruses. Safest Choice™ eggs are safe to use in any recipe Net Shipping Weight 1350 lbs. Full Pallet (22.5 lbs/cs) even undercooked or raw. Discover: Case Dimensions (LxWxH) 12x12x13.5 • Nature’s most versatile comfort food tasty, nutritious eggs Case Cube 1.15 • A simple recipe ingredient customers crave Dozens per Case 15 • Farm fresh, wholesome flavor Shelf Life Sell By 67 Days (Exceptions: CA Sell By 30 Days, • A top culinary performer adaptable to all menus and recipes Best Before 67 Days. IL Sell by 45 Days) • A perfect fit in any production/service system Product Codes UPC code Description Pack Size A Top Culinary Performer! 7-63514-41430-4 Medium – Pasteurized Shell Eggs 1/15 Dozen ™ Use Safest Choice Pasteurized Shell Eggs in: 7-63514-41420-5 Large – Pasteurized Shell Eggs 1/15 Dozen • Retail breakfast/all-day breakfast service 7-63514-41410-6 X-Large – Pasteurized Shell Eggs 1/15 Dozen • Grill/cook to order 7-63514-42420-4 Large – Pasteurized Cage 1/15 Dozen • Ethnic specialties (e.g., huevos rancheros) Cage Free Shell Eggs Free • Room service • Retail service Cage Free and Certified Humane Safest Choice™ Cage Free Eggs are Certified • Sandwich stations Humane,® meeting rigid animal welfare • Casseroles & quiches standards towards the goal of improving the lives of millions of farm animals. -
Chapter 7 Food Safety Fact Sheets
Food Safety Fact Sheets Kitchen Food Safety Tips How safe is your kitchen? Did you know that most foodborne illness results from poor food handling at home? Your kitchen could be a high risk environment. Bacteria can thrive in food that is improperly stored or handled. Reduce the risks by following these tips from Canada’s food safety experts. Play it “food safe” in your kitchen! Get off to a CLEAN start! • Handwashing is one of the best ways to prevent the spread of foodborne illness. Do you wash your hands for at least 20 seconds with soap and warm water before and after handling food? Wash again when you switch from one food to another. • Are your countertops and utensils clean and sanitized? Sanitizing reduces bacteria and can prevent foodborne illness. It’s important to thoroughly clean everything that comes in contact with your hands or your food! Don’t forget about kitchen cloths . faucet handles . sink drains . garbage disposals . can opener blades . refrigerator handles . small appliances . utensils, and so on. BLEACH SANITIZER • Combine 2 mL (1/2 tsp) of liquid chlorine for every 1 litre of H20 to make a disinfection solution of 100 mg/lor 100ppm. • After cleaning, spray sanitizer on the surface/utensil and let stand briefly. • Rinse with lots of clean water, and air dry (or use clean towels). Eight quick tips for the kitchen (at home, work, school, etc.) 1. Keep separate cutting boards for raw meat, poultry and seafood and a different one for ready-to-eat and cooked foods. Clean and sanitize cutting boards after each use. -

Consumer Advisory
County of Santa Clara Department of Environmental Health Consumer Protection Division 1555 Berger Drive, Suite 300 San Jose, CA 95112-2716 (408)918-3400 FAX (408)258-5891 www.EHinfo.org/CPD CONSUMER ADVISORY Introduction The California Retail Food Code (CalCode) Section 114093 requires a “Consumer Advisory” for animal foods, including beef, eggs, fish, lamb, milk, pork, poultry, or shellfish served or sold raw, undercooked, or without otherwise being processed to eliminate pathogens, either in ready-to-eat form or as an ingredient in another ready-to- eat food. A retail food facility permit holder shall inform consumers of the significantly increased risk of consuming such foods. CalCode requires the permit holder to provide a “Consumer Advisory” using a DISCLOSURE and REMINDER. 1. A DISCLOSURE is a clearly written statement that includes either: a. A description of the animal-derived foods, such as “oysters on the half shell (raw oysters)”, “raw-egg Caesar salad” and “hamburger (can be cooked to order).” or b. Identification of the animal derived foods marked by an asterisk (*) directing to a footnote that states that the items are served raw or undercooked, or contain or may contain raw or undercooked ingredients. 2. A REMINDER is a written statement that identifies the animal-derived foods by an asterisk that marks a footnote that includes either of the following disclosure statements: a. Written information regarding the safety of these food items is available upon request. b. Consuming raw or undercooked meats, poultry, seafood, shellfish, or eggs may increase your risk of foodborne illness, especially if you have certain medical conditions. -

Ostrich Production Systems Part I: a Review
11111111111,- 1SSN 0254-6019 Ostrich production systems Food and Agriculture Organization of 111160mmi the United Natiorp str. ro ucti s ct1rns Part A review by Dr M.M. ,,hanawany International Consultant Part II Case studies by Dr John Dingle FAO Visiting Scientist Food and , Agriculture Organization of the ' United , Nations Ot,i1 The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. M-21 ISBN 92-5-104300-0 Reproduction of this publication for educational or other non-commercial purposes is authorized without any prior written permission from the copyright holders provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without written permission of the copyright holders. Applications for such permission, with a statement of the purpose and extent of the reproduction, should be addressed to the Director, Information Division, Food and Agriculture Organization of the United Nations, Viale dells Terme di Caracalla, 00100 Rome, Italy. C) FAO 1999 Contents PART I - PRODUCTION SYSTEMS INTRODUCTION Chapter 1 ORIGIN AND EVOLUTION OF THE OSTRICH 5 Classification of the ostrich in the animal kingdom 5 Geographical distribution of ratites 8 Ostrich subspecies 10 The North -

Safe Handling of Eggs
HOME & GARDEN INFORMATION 1-888-656-9988 HGIC 3507 http://hgic.clemson.edu CENTER Safe Handling of Eggs SHELL EGG SAFETY recipes in which the raw egg ingredients are not Eggs can be a part of a healthy diet. However, they cooked. These egg-based recipes should be updated are perishable just like raw meat, poultry and fish. to start with a cooked base or so that commercially To be safe, they must be properly stored, handled prepared pasteurized eggs or egg substitutes are and cooked. used. Use a thermometer and make sure the temperature of the cooked base reaches 160 °F. Concern for Egg Safety: Some unbroken fresh shell eggs may contain Salmonella enteritidis Buy Clean Eggs from a Refrigerator Display bacteria that can cause foodborne illness. While the Case: Do not purchase eggs anywhere that are not number of eggs affected is quite small, there have refrigerated. Any bacteria present in the egg can been some scattered outbreaks in the last few years. grow quickly if stored at room temperature. At the Currently the government, the egg industry and the store, choose Grade A or AA eggs with clean, scientific community are working together to solve uncracked shells. the problem. Safe Storage of Eggs at Home: Take eggs straight Researchers say that if present, the Salmonella home and store them immediately in the refrigerator enteritidus bacteria are usually in the yolk or at 40 °F or slightly below. Store them in the gro- “yellow.” But they cannot rule out the bacteria cery carton in the coldest part of the refrigerator, not being in egg whites. -

Mind Your Eggs & Veggies
MIND YOUR EGGS & VEGGIES Nutrition for Cognitive Health Presented by Taylor C. Wallace, PhD, CFS, FACN And Chef Abbie Gellman, MS, RD, CDN Spread the Fruit and Veggie Love #haveaplant @fruits_veggies @fruitsandveggies @fruitsandveggies © 2020 Produce for Better Health Foundation 2 Our Purpose The Produce for Better Health Foundation (PBH), a 501(c)3, is the only national non-profit organization committed to helping people live happier, healthier lives by eating more fruits and vegetables in all their glorious forms every day. © 20202019 Produce for Better Health Foundation 3 Our Movement Research shows, rather than a prescriptive recommendation to eat a certain amount of fruits and vegetables each day, consumers (particularly Gen Z and Millennials) want actionable, realistic and FUN approaches that make eating fruits and vegetables easy, helping them feel confident, happy and healthy. That’s where PBH’s Have A Plant® movement comes in. It’s a way to tap into the emotional connection consumers have to the fruit and vegetable eating experience while inspiring long-term, sustainable behavior change. And it does so with a no-nonsense approach that’s simple, understandable, and, importantly for this audience, non-prescriptive. © 2020 Produce for Better Health Foundation 4 Moderator Wendy Reinhardt Kapsak, MS, RDN © 2020 Produce for Better Health Foundation 5 Content Series: Eggs: Veggies’ Reliable BFF © 2020 Produce for Better Health Foundation 6 Adding Eggs to Enhance the Benefits of Produce Taylor C. Wallace, PhD, CFS, FACN Think Healthy Group, Inc. George Mason University © 2020 Produce for Better Health Foundation 7 Disclosures • Think Healthy Group, Inc. • George Mason University • The Dr. -

2020 Front Cover
INDIANA STATE POULTRY ASSOCIATION PRESENTS: 2020 POULTRY INFOMATION BOOKLET THIS BOOKLET CONTAINS: Test Your Knowledge - Poultry Quiz (Inside Front Cover) Poultry Terms Quick Reference Guide (Pg. 1) Getting Started with a Home Poultry Flock (Pg. 2) Common Egg Shell Quality Problems (Pg. 5) Choosing a Chicken Breed: Eggs, Meat or Exhibition (Pg. 6) Stay Healthy When Working with Farm Animals (Pg. 9) Exhibition Poultry (Pg. 10) Proper Handling of Eggs: From Hen to Consumption (Pg. 12) Don’t Get Caught Without These Forms (Pg. 17) Avian Influenza Findings Emphasize the Need for Good Biosecurity (Pg. 18) Backyard Biosecurity Self Evaluation (Pg. 20) Indiana Test Twelve Flock Evaluation (Inside Back Cover) This Booklet is designed to be a quick resource for raising poultry. For additional information go to www.INpoultry.com INDIANA STATE POULTRY ASSOCIATION PURDUE UNIVERSITY, ANIMAL SCIENCES 270 SOUTH RUSSELL STREET • WEST LAFAYETTE, IN 47907 765-494-8517 • [email protected] • WWW.INPOULTRY.COM TEST YOUR KNOWLEDGE - POULTRY QUIZ THINK YOU KNOW POULTRY? ANSWER THESE TEN QUESTIONS FOR A CHANCE TO WIN A PRIZE. ALL ANSWERS CAN BE FOUND THROUGHOUT THIS BOOKLET. 1. If chicks move away from the heat source in their brooding area and appear to be drowsy, then the temperature is most likely too ________ . 2. What might be the cause of a misshapen egg? a. Immature shell gland b. Disease c. Stress d. Overcrowding e. All of the above 3. Chicken breeds with _______ ear lobes lay white eggs while chicken breeds with ______ ear lobes lay brown eggs. 4. Anyone can get sick from farm animals, but what groups of people are more likely to have a serious illness? a. -

An Introduction to Morphology of the Reproductive System and Anatomy of Hen's
J. Life Earth Sci., Vol. 8: 1-10, 2013 ISSN 1990-4827 http://banglajol.info.index.php/JLES © 2013, JLES, RU AN INTRODUCTION TO MORPHOLOGY OF THE REPRODUCTIVE SYSTEM AND ANATOMY OF HEN’S EGG Md. Anisur Rahman Department of Zoology, University of Rajshahi, Rajshahi-6205, Bangladesh e-mail: [email protected] Abstract: The present study was designed to investigate the morphology of reproductive system and egg anatomy of the domestic hen (Gallus domesticus L.). The system consists of oviduct and ovary. The oviduct consists of infundibulum, magnum, isthmus, uterus and vagina which are sole distributor for making nutrition enriched egg. The anatomy of egg revealed that there are calcareous eggshell, shell membranes, egg white, vitelline membrane, egg yolk, and germinal disc. The fertilized egg showed a concentric circle around the nucleus known as blastoderm that contained area pellucida and area opaca whereas an unfertilized egg showed nucleus as white spot (blastodisc). Primordial germ cells (PGCs) are progenitor cells of ova and spermatozoa and they originate from the epiblast of the central part of the area pellucida. The microscopic structure of eggshell rendered leathery cuticle, fibrous matrix and shell membranes. The egg protects itself by its own mechanism from being injured and provides a complete diet for the developing embryo. Key words: Ovary, oviduct, egg, blastodisc, eggshell mvivsk: eZ©gvb Aa¨qbwU gyiwMi (Gallus domesticus L.) cÖRbbZ‡š¿i A½ms¯’vb Ges wW‡gi A½e¨e‡”Q` AbymÜvb Kivi Rb¨ Av‡jLb Kiv n‡q‡Q| cÖRbbZš¿wU‡Z wW¤^bvjx -
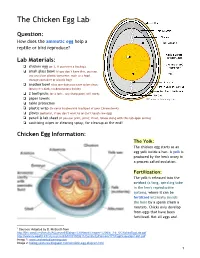
The Chicken Egg Lab 1
The Chicken Egg Lab1 Question: How does the amniotic egg help a reptile or bird reproduce? Lab Materials: ❏ chicken egg (or 2, if you want a backup) ❏ small glass bowl (if you don’t have this, you can use any clear plastic container, such as a food storage container or plastic bag) ❏ another bowl (this one does not have to be clear; ideally it’s dark, no decorations inside) ❏ 2 toothpicks (or a fork… any sharp point will work) ❏ paper towels ❏ table protection ❏ plastic wrap (to cover keyboard & trackpad of your Chromebook) ❏ gloves (optional, if you don’t want to or can’t touch raw egg) ❏ pencil & lab sheet (if you can print, print; if not, follow along with this tab open online) ❏ sanitizing wipes or cleaning spray, for cleanup at the end! Chicken Egg Information: The Yolk: The chicken egg starts as an egg yolk inside a hen. A yolk is produced by the hen's ovary in a process called ovulation. Fertilization: The yolk is released into the oviduct (a long, spiraling tube in the hen's reproductive system), where it can be fertilized internally (inside the hen) by a sperm (from a rooster). Chicks only develop from eggs that have been fertilized. Not all eggs are! 1 Sources: Adapted by D. McBeath from http://ljhs.sandi.net/faculty/AQuesnell/Biology%20Notes/Chapter%206/6_3-6_5/ChickenEggLab.pdf http://www.newpaltz.k12.ny.us/cms/lib/NY01000611/Centricity/Domain/171/EggDissectionLab1.pdf Image 1: www.enchantedlearning.com Image 2: biology-pictures.blogspot.com/amniotic-egg-diagram.html 1 The Egg White (albumin): The yolk continues down the oviduct (whether or not it is fertilized) and is covered with a membrane, structural fibers, and layers of albumin protein (the egg white). -

Real Eggs. with Real Advantages
Real Eggs. With Real Advantages. Still need to crack? We have a safer solution. Refrigerated Liquid Eggs vs. Traditional Shell Eggs With Papetti’s® liquid eggs, you still get real, classic, from-the-shell eggs. We’ve simply taken those eggs and cracked them for you. Plus, they are pasteurized and conveniently packaged to reduce the stress on your kitchen and increase your peace of mind! Papetti’s® Liquid Eggs VS Traditional Shell Eggs Taste Real Eggs! Real Eggs! So they taste like real eggs. So they taste like real eggs. Convenience Always Ready to Use. No Cracking. No Mess! Cracking Required. Mess Likely. Ready to pour straight from the cooler. Cracking and stirring takes up precious time. Shelf Life of Up to 98 Days Shelf Life of Up to 30 Days Last over 3 times longer than shell eggs. Constantly having to worry about inventory. Condensed Packaging (i.e. Cartons & Bags) Bulky Cases of Flats & Cartons Requires only 1/3 the cooler space vs. shell eggs. Requires considerably more cooler space. Safe & Efficient Operating Procedures Tedious Safe-Handling Procedures & Breakage Pasteurized eggs allow you to quickly move from one task to the next Sanitizing stations, washing hands & changing gloves all slow down prep. without having to deal with time consuming safety procedures. Pooling eggs is restricted. Not to mention, dropped, cracked & rotten eggs. Ready-to-Go Options One Option to Start with Every Time Choose from whole eggs, whites, yolks and scrambled egg mixes! Requires cracking, separating, whisking, and more. Savings Save Time. Save Money. Takes Time. Takes Money.