006-2010 Almeida Et Al.Pmd
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CHECKLIST and BIOGEOGRAPHY of FISHES from GUADALUPE ISLAND, WESTERN MEXICO Héctor Reyes-Bonilla, Arturo Ayala-Bocos, Luis E
ReyeS-BONIllA eT Al: CheCklIST AND BIOgeOgRAphy Of fISheS fROm gUADAlUpe ISlAND CalCOfI Rep., Vol. 51, 2010 CHECKLIST AND BIOGEOGRAPHY OF FISHES FROM GUADALUPE ISLAND, WESTERN MEXICO Héctor REyES-BONILLA, Arturo AyALA-BOCOS, LUIS E. Calderon-AGUILERA SAúL GONzáLEz-Romero, ISRAEL SáNCHEz-ALCántara Centro de Investigación Científica y de Educación Superior de Ensenada AND MARIANA Walther MENDOzA Carretera Tijuana - Ensenada # 3918, zona Playitas, C.P. 22860 Universidad Autónoma de Baja California Sur Ensenada, B.C., México Departamento de Biología Marina Tel: +52 646 1750500, ext. 25257; Fax: +52 646 Apartado postal 19-B, CP 23080 [email protected] La Paz, B.C.S., México. Tel: (612) 123-8800, ext. 4160; Fax: (612) 123-8819 NADIA C. Olivares-BAñUELOS [email protected] Reserva de la Biosfera Isla Guadalupe Comisión Nacional de áreas Naturales Protegidas yULIANA R. BEDOLLA-GUzMáN AND Avenida del Puerto 375, local 30 Arturo RAMíREz-VALDEz Fraccionamiento Playas de Ensenada, C.P. 22880 Universidad Autónoma de Baja California Ensenada, B.C., México Facultad de Ciencias Marinas, Instituto de Investigaciones Oceanológicas Universidad Autónoma de Baja California, Carr. Tijuana-Ensenada km. 107, Apartado postal 453, C.P. 22890 Ensenada, B.C., México ABSTRACT recognized the biological and ecological significance of Guadalupe Island, off Baja California, México, is Guadalupe Island, and declared it a Biosphere Reserve an important fishing area which also harbors high (SEMARNAT 2005). marine biodiversity. Based on field data, literature Guadalupe Island is isolated, far away from the main- reviews, and scientific collection records, we pres- land and has limited logistic facilities to conduct scien- ent a comprehensive checklist of the local fish fauna, tific studies. -

Environmental DNA Reveals the Fine-Grained and Hierarchical
www.nature.com/scientificreports OPEN Environmental DNA reveals the fne‑grained and hierarchical spatial structure of kelp forest fsh communities Thomas Lamy 1,2*, Kathleen J. Pitz 3, Francisco P. Chavez3, Christie E. Yorke1 & Robert J. Miller1 Biodiversity is changing at an accelerating rate at both local and regional scales. Beta diversity, which quantifes species turnover between these two scales, is emerging as a key driver of ecosystem function that can inform spatial conservation. Yet measuring biodiversity remains a major challenge, especially in aquatic ecosystems. Decoding environmental DNA (eDNA) left behind by organisms ofers the possibility of detecting species sans direct observation, a Rosetta Stone for biodiversity. While eDNA has proven useful to illuminate diversity in aquatic ecosystems, its utility for measuring beta diversity over spatial scales small enough to be relevant to conservation purposes is poorly known. Here we tested how eDNA performs relative to underwater visual census (UVC) to evaluate beta diversity of marine communities. We paired UVC with 12S eDNA metabarcoding and used a spatially structured hierarchical sampling design to assess key spatial metrics of fsh communities on temperate rocky reefs in southern California. eDNA provided a more‑detailed picture of the main sources of spatial variation in both taxonomic richness and community turnover, which primarily arose due to strong species fltering within and among rocky reefs. As expected, eDNA detected more taxa at the regional scale (69 vs. 38) which accumulated quickly with space and plateaued at only ~ 11 samples. Conversely, the discovery rate of new taxa was slower with no sign of saturation for UVC. -

Atividade De Limpeza E Clientes De Elacatinus Figaro (Pisces: Gobiidae) Nos Recifes De Coral Dos Parrachos De Muriú, Nordeste Do Brasil
Atividade de limpeza e clientes de Elacatinus figaro (Pisces: Gobiidae) nos recifes de coral dos Parrachos de Muriú, Nordeste do Brasil Campos, C.E.C. & Sá-Oliveira, J.C. Biota Neotrop. 2011, 11(1): 47-52. On line version of this paper is available from: http://www.biotaneotropica.org.br/v11n1/en/abstract?article+bn01011012011 A versão on-line completa deste artigo está disponível em: http://www.biotaneotropica.org.br/v11n1/pt/abstract?article+bn01011012011 Received/ Recebido em 30/06/2010 - Revised/ Versão reformulada recebida em 14/12/2010 - Accepted/ Publicado em 11/01/2011 ISSN 1676-0603 (on-line) Biota Neotropica is an electronic, peer-reviewed journal edited by the Program BIOTA/FAPESP: The Virtual Institute of Biodiversity. This journal’s aim is to disseminate the results of original research work, associated or not to the program, concerned with characterization, conservation and sustainable use of biodiversity within the Neotropical region. Biota Neotropica é uma revista do Programa BIOTA/FAPESP - O Instituto Virtual da Biodiversidade, que publica resultados de pesquisa original, vinculada ou não ao programa, que abordem a temática caracterização, conservação e uso sustentável da biodiversidade na região Neotropical. Biota Neotropica is an eletronic journal which is available free at the following site http://www.biotaneotropica.org.br A Biota Neotropica é uma revista eletrônica e está integral e gratuitamente disponível no endereço http://www.biotaneotropica.org.br Biota Neotrop., vol. 11, no. 1 Atividade de limpeza e clientes de Elacatinus figaro (Pisces: Gobiidae) nos recifes de coral dos Parrachos de Muriú, Nordeste do Brasil Carlos Eduardo Costa Campos1,2 & Júlio César Sá-Oliveira1 1 Laboratório de Zoologia, Departamento de Ciências Biológicas, Universidade Federal do Amapá – UNIFAP, Rod. -
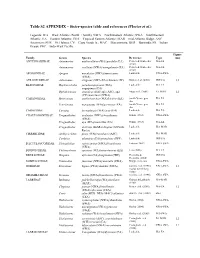
Table S2 APPENDIX – Sister-Species Table and References (Floeter Et Al.)
Table S2 APPENDIX – Sister-species table and references (Floeter et al.) Legends: WA = West Atlantic (North + South); NWA = Northwestern Atlantic; SWA = Southwestern Atlantic; EA = Eastern Atlantic; TEA = Tropical Eastern Atlantic; MAR = mid-Atlantic Ridge; ASC = Ascension; SHE = St. Helena; CV = Cape Verde Is.; MAC = Macaronesia; BER = Bermuda; IO = Indian Ocean; IWP = Indo-West Pacific. Figure Family Genus Species Reference Type (ms) ANTENNARIIDAE Antennarius multiocellatus (WA)/pardalis (EA) Pietsch & Grobecker WA-EA (1987) Antennarius ocellatus (NWA)/senegalensis (EA) Pietsch & Grobecker WA-EA (1987) APOGONIDAE Apogon maculatus (NWA)/americanus Look alike NWA-SWA (SWA) AULOSTOMIDAE Aulostomus strigosus (SWA-EA)/chinensis (IP) Bowen et al. (2001) IWP-EA 12 BLENNIIDAE Hypleurochilus pseudoaequipinnis (WA)/ Look alike WA-EA aequipinnis (EA) Ophioblennius atlanticus (EA)/ sp1 (ASC), sp2 Muss et al. (2001) EA-MAR 12 (CV)/macclurei (NWA) CARANGIDAE Hemicaranx amblyrhynchus (WA)/bicolor (EA) Smith-Vaniz, pers WA-EA obs. Trachinotus marginatus (WA)/goreensis (EA) Smith-Vaniz, pers WA-EA obs. CARAPIDAE Carapus bermudensis (WA)/acus (EA) Look alike WA-EA CHAETODONTIDAE Prognathodes aculeatus (NWA)/brasiliensis Hubbs (1963) NWA-SWA (SWA) Prognathodes aya (WA)/marcellae (EA) Hubbs (1963) WA-EA Prognathodes dichrous (MAR)/obliquus (St Paul's Look alike WA-MAR Rocks) CIRRHITIDAE Amblycirrhitus pinos (WA)/earnshawi (ASC) Look alike WA-MAR Cirrhitus atlanticus (EA)/pinnulatus (IWP) Look alike IWP-EA DACTYLOSCOPIDAE Platygillellus rubrocinctus (NWA)/brasiliensis Feitoza (2002) NWA-SWA (SWA) DIODONTIDAE Chilomycterus spinosus (WA)/mauretanicus (EA) Leis (2006) WA-EA DREPANIDAE Drepane africana (EA)/longimana (IWP) Heemstra & IWP-EA Heemstra (2004) GOBIESOCIDAE Tomicodon fasciatus (NWA)/australis (SWA) Briggs, pers com NWA-SWA GOBIIDAE Elacatinus figaro (SWA)/randalli (NWA) Sazima et al. -

The Global Trade in Marine Ornamental Species
From Ocean to Aquarium The global trade in marine ornamental species Colette Wabnitz, Michelle Taylor, Edmund Green and Tries Razak From Ocean to Aquarium The global trade in marine ornamental species Colette Wabnitz, Michelle Taylor, Edmund Green and Tries Razak ACKNOWLEDGEMENTS UNEP World Conservation This report would not have been The authors would like to thank Helen Monitoring Centre possible without the participation of Corrigan for her help with the analyses 219 Huntingdon Road many colleagues from the Marine of CITES data, and Sarah Ferriss for Cambridge CB3 0DL, UK Aquarium Council, particularly assisting in assembling information Tel: +44 (0) 1223 277314 Aquilino A. Alvarez, Paul Holthus and and analysing Annex D and GMAD data Fax: +44 (0) 1223 277136 Peter Scott, and all trading companies on Hippocampus spp. We are grateful E-mail: [email protected] who made data available to us for to Neville Ash for reviewing and editing Website: www.unep-wcmc.org inclusion into GMAD. The kind earlier versions of the manuscript. Director: Mark Collins assistance of Akbar, John Brandt, Thanks also for additional John Caldwell, Lucy Conway, Emily comments to Katharina Fabricius, THE UNEP WORLD CONSERVATION Corcoran, Keith Davenport, John Daphné Fautin, Bert Hoeksema, Caroline MONITORING CENTRE is the biodiversity Dawes, MM Faugère et Gavand, Cédric Raymakers and Charles Veron; for assessment and policy implemen- Genevois, Thomas Jung, Peter Karn, providing reprints, to Alan Friedlander, tation arm of the United Nations Firoze Nathani, Manfred Menzel, Julie Hawkins, Sherry Larkin and Tom Environment Programme (UNEP), the Davide di Mohtarami, Edward Molou, Ogawa; and for providing the picture on world’s foremost intergovernmental environmental organization. -

Development of Larval Fish Rearing Techniques and Nutrient Requirements for the Green Mandarin, Synchiropus Splendidus: a Popular Marine Ornamental Fish
ResearchOnline@JCU This file is part of the following reference: Shao, Luchang (2016) Development of larval fish rearing techniques and nutrient requirements for the green mandarin, Synchiropus splendidus: a popular marine ornamental fish. PhD thesis, James Cook University. Access to this file is available from: http://researchonline.jcu.edu.au/47308/ The author has certified to JCU that they have made a reasonable effort to gain permission and acknowledge the owner of any third party copyright material included in this document. If you believe that this is not the case, please contact [email protected] and quote http://researchonline.jcu.edu.au/47308/ Development of larval fish rearing techniques and nutrient requirement for the green mandarin, Synchiropus splendidus: a popular marine ornamental fish Thesis submitted by Luchang Shao (MSc) in September 2016 For the degree of Doctor of Philosophy In the College of Marine and Environmental Science James Cook University Declaration on Ethics The research presented and reported in this thesis was conducted within the guidelines for research ethics outlined in the National Statement on Ethics Conduct in Research Involving Human (1999), the Joint NHMRC/AVCC Statement and Guidelines on Research Practice (1997), the James Cook University Policy on Experimentation Ethics Standard Practices and Guidelines (2001), and the James Cook University Statement and Guidelines on Research Practice (2001). The proposed research methodology received clearance from the James Cook University Experimentation Ethics Review Committee. Approval numbers: A1851; Principal investigator: Luchang Shao; Finish date: September 30, 2015 i Statement of contribution of others Financial support for this study was provided by Graduate Research School of James Cook University, JCU Postgraduate Research Scholarship. -

Reef Fisheries and Underwater Surveys Indicate Overfishing of a Brazilian Coastal Island
Research Letters Natureza & Conservação 8(2):151-159, December 2010 Copyright© 2010 ABECO Handling Editor: Sergio R. Floeter Brazilian Journal of Nature Conservation doi: 10.4322/natcon.00802008 Reef Fisheries and Underwater Surveys Indicate Overfishing of a Brazilian Coastal Island Hudson Tercio Pinheiro*, Jean-Christophe Joyeux & Agnaldo Silva Martins Departamento de Oceanografia e Ecologia, Universidade Federal do Espírito Santo, Vitória, ES, Brasil Abstract The preoccupation about fishing effects on marine ecosystems has increased sharply over the last three decades. However, little is known about the impact of multi-gear artisanal and recreational fisheries on the structure of local reef fish communities in Brazil. Fishing activities around a Brazilian coastal island were monitored while reef fish density was censused during underwater surveys (UVC). The links between frequency of capture, intensity at which species are wished and UVC density were explored. Species were classified according to their frequency of capture as regular, occasional and rare, and classified according to the intensity at which they are wished (based on size and price), as highly targeted, average and non-targeted species. Ninety-seven species were caught by fishing, the majority of them being either rarely caught or non-targeted. Nineteen species were highly targeted but rarely caught. The highly targeted species showed extremely low density in the UVC. These results put in question the sustainability of the local fishing activities. The predominance of non-targeted species in the catches and in the reefs environment studied supports the expectation that these species will be more and more captured, thus collaborating to further change the structure of the reef community. -

Guide to the Coastal Marine Fishes of California
STATE OF CALIFORNIA THE RESOURCES AGENCY DEPARTMENT OF FISH AND GAME FISH BULLETIN 157 GUIDE TO THE COASTAL MARINE FISHES OF CALIFORNIA by DANIEL J. MILLER and ROBERT N. LEA Marine Resources Region 1972 ABSTRACT This is a comprehensive identification guide encompassing all shallow marine fishes within California waters. Geographic range limits, maximum size, depth range, a brief color description, and some meristic counts including, if available: fin ray counts, lateral line pores, lateral line scales, gill rakers, and vertebrae are given. Body proportions and shapes are used in the keys and a state- ment concerning the rarity or commonness in California is given for each species. In all, 554 species are described. Three of these have not been re- corded or confirmed as occurring in California waters but are included since they are apt to appear. The remainder have been recorded as occurring in an area between the Mexican and Oregon borders and offshore to at least 50 miles. Five of California species as yet have not been named or described, and ichthyologists studying these new forms have given information on identification to enable inclusion here. A dichotomous key to 144 families includes an outline figure of a repre- sentative for all but two families. Keys are presented for all larger families, and diagnostic features are pointed out on most of the figures. Illustrations are presented for all but eight species. Of the 554 species, 439 are found primarily in depths less than 400 ft., 48 are meso- or bathypelagic species, and 67 are deepwater bottom dwelling forms rarely taken in less than 400 ft. -

Reef Fish Associations with Sea Urchins in an Atlantic Oceanic Island
Mar Biodiv (2018) 48:1833–1839 DOI 10.1007/s12526-017-0677-4 ORIGINAL PAPER Reef fish associations with sea urchins in an Atlantic oceanic island Vinicius J. Giglio1,2 & Maria L. F. Ternes3 & Moysés C. Barbosa2 & César A. M. M. Cordeiro2 & Sergio R. Floeter4 & Carlos E. L. Ferreira2 Received: 19 November 2016 /Revised: 27 February 2017 /Accepted: 28 February 2017 /Published online: 16 March 2017 # Senckenberg Gesellschaft für Naturforschung and Springer-Verlag Berlin Heidelberg 2017 Abstract Many reef fish are known to be associated with Introduction particular microhabitats that provide food and refuge, such as branching corals, gorgonians, macroalgal beds and sea ur- Habitat structure and complexity are known to influence reef chins. We investigate the association of reef fishes with the fish abundance and diversity at various spatial scales from long-spined sea urchin Diadema antillarum in shallow reefs micro to mega habitats (Roberts and Ormond 1987; Ferreira of Trindade Island, southeastern Brazil. A total of 1283 fish et al. 2001;Rogersetal.2014). In reef environments, many individuals from seven families and nine species were associ- fish species are known to be associated with particular habi- ated with 495 sea urchins. Sea urchins provide important shel- tats, mostly due to optimal refuge against predators and avail- ter especially for juveniles of the Noronha wrasse, ability of food (Dahlgren and Eggleston 2000). Many organ- Thalassoma noronhanum. Larger fishes were found at higher isms are known to modify the environment or create micro- densities associated to sea urchins with larger spines. At reefs habitats, being described as ecosystem engineers (Jones et al. -

Cryptobenthic Fish As Clients of French Angelfish Pomacanthus Paru (Pomacanthidae) During Cleaning Behaviour Cláudio L
Sampaio et al. Marine Biodiversity Records (2017) 10:8 DOI 10.1186/s41200-017-0109-y MARINERECORD Open Access Cryptobenthic fish as clients of french angelfish Pomacanthus paru (Pomacanthidae) during cleaning behaviour Cláudio L. S. Sampaio1, Miguel Loiola2,3, Liliana P. Colman6, Diego V. Medeiros7, Juan Pablo Quimbayo8, Ricardo J. Miranda2,4, José Amorim Reis-Filho2,4,5* and José de Anchieta C. C. Nunes2,4 Abstract The French angelfish Pomacanthus paru (Pomacanthidae) is recognised as an important cleaner in tropical reef environments, yet its clients remain relatively undescribed in the literature. Here, we report observations of their cleaning behaviour when interacting with different species of cryptobenthic fish clients. The study was conducted in Bahia state, northeast Brazil. In this region, French angelfish were seen cleaning four different species of cryptobenthic species, respectively, Coryphopterus glaucofraenum, Scorpaena plumieri, Labrisomus cricota,andScartella cristata.These records show the broad spectra of clients that cleaners interact with in coral reef systems, as well as give important insights into the poorly known cryptobenthic fishes habits and ecology. Keywords: Reef fish, Cleaner fish, Facultative cleaner, Tropical rocky shores, Brazil Introduction et al. 2005) species worldwide, the majority being facul- Cleaning symbiosis has been reported as one of the most tative cleaners. important interspecific interactions in reef environments In Brazilian coastal waters, there is only one species (Côté and Molloy 2003) and it contributes to increased known to act as an obligatory cleaner (the endemic bar- reef fish diversity in such systems (Grutter et al. 2003). ber goby Elacatinus figaro Sazima, Moura & Rosa 1997). Cleaner species can either be obligatory or facultative. -

Luizjunior Osmarjose M.Pdf
“COMPOSIÇÃO E ESTRUTURA DA COMUNIDADE DE PEIXES RECIFAIS EM RELAÇÃO A QUATRO VARIÁVEIS AMBIENTAIS NO PARQUE ESTADUAL MARINHO DA LAJE DE SANTOS, ESTADO DE SÃO PAULO” 1 Dedico este trabalho ao meu filho, Pedro Henrique Sanches Luiz e a minha esposa, Alessandra Sanches Luiz. Um amor maior que o mar. 2 “To be a naturalist is better than to be a king” William Beebe, 1893 3 AGRADECIMENTOS A minha família, Alessandra e Pedro Henrique, pelo apoio e amor incondicional e por terem suportado os momentos em que estive ausente em decorrência deste trabalho. Sergio Ricardo Floeter, Carlos Eduardo Leite Ferreira e João Luiz Gasparini por serem ao mesmo tempo amigos, professores, irmãos, mestres, companheiros e por serem os principais responsáveis por hoje eu seguir esta carreira, sou eternamente grato. Ao Professor Ivan Sazima pela confiança, orientação e imenso apoio durante todas as etapas deste trabalho e de outros trabalhos realizados. João Paulo Krajewski, Angela Correa da Silva e Roberta Martini Bonaldo pela imensa amizade e por me acolherem como um membro da família quando precisei de abrigo em Campinas. Aos companheiros do Instituto Laje Viva: Guilherme Kodja, Ana Paula Balboni Pinto, Ricardo Coelho, Paula Romano, Cristiane Morgado, Vilma Lira, José Eduardo Guariglia Filho, Andreia Gondim, Luis Fernando Waib, Big Paul, Lucia Silva e Rogério Brito pela amizade, ajuda no campo e apoio logístico. Ao amigo Clóvis B. de Carvalho, por ter me ensinado a amar a “Grande Pedra Mágica”. Carlo Leopoldo B. Francini, Luis Fernando Cassino, Maurício Andrade, Augusto Valente, Ivan Cavas, Armando de Luca Jr., Alfredo Carvalho-Filho, Renata Linger, Lara Cheidde e Rafael Leite pelas inúmeras fotografias cedidas para a utilização neste trabalho. -

Variation in the Presence and Cause of Density-Dependent Mortality in Three Species of Reef Fishes
Ecology, 81(9), 2000, pp. 2416±2427 q 2000 by the Ecological Society of America VARIATION IN THE PRESENCE AND CAUSE OF DENSITY-DEPENDENT MORTALITY IN THREE SPECIES OF REEF FISHES GRAHAM E. FORRESTER1 AND MARK A. STEELE Department of Organismic Biology, Ecology, and Evolution, University of California, 621 Charles E. Young Drive South, Los Angeles, California 90095-1606, USA Abstract. Determining the mechanisms by which natural populations are regulated is a key issue in ecology. Identifying the biological causes of density dependence has, however, proved dif®cult in many systems. In this study we tested whether adults of three species of reef ®sh (all gobies) suffered density-dependent mortality, and whether the density- dependent component of mortality was caused by predation. We used ®eld experiments to test for density dependence in each prey species, manipulating the presence of predators and prey density in a factorial design. Prey were stocked on replicate patches of reef constructed of natural materials, with each reef receiving a different density of gobies. Predatory ®shes were excluded from half of the reefs using a combination of removals and exclusion cages. Survival of the ®rst species, Lythrypnus dalli, was high and density- independent on reefs free of predators, but declined rapidly with increasing density on reefs to which predators had access. Density dependence in L. dalli was thus a result of mortality in¯icted by predatory ®shes. In the second species, Coryphopterus nicholsii, predators caused a large reduction in the survival in one experiment but had a negligible effect in a second experiment. More importantly, though, survival of C.