Development of Larval Fish Rearing Techniques and Nutrient Requirements for the Green Mandarin, Synchiropus Splendidus: a Popular Marine Ornamental Fish
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Histochemical Characteristics of Macrophages of Butterfly Splitfin Ameca Splendens
e-ISSN 1734-9168 Folia Biologica (Kraków), vol. 67 (2019), No 1 http://www.isez.pan.krakow.pl/en/folia-biologica.html https://doi.org/10.3409/fb_67-1.05 Histochemical Characteristics of Macrophages of Butterfly Splitfin Ameca splendens Ewelina LATOSZEK, Maciej KAMASZEWSKI , Konrad MILCZAREK, Kamila PUPPEL, Hubert SZUDROWICZ, Antoni ADAMSKI, Pawe³ BURY-BURZYMSKI, and Teresa OSTASZEWSKA Accepted March 18, 2019 Published online March 29, 2019 Issue online March 29, 2019 Original article LATOSZEK E., KAMASZEWSKI M., MILCZAREK K., PUPPEL K., SZUDROWICZ H., ADAMSKI A., BURY-BURZYMSKI P., OSTASZEWSKA T. 2019. Histochemical characteristics of macrophages of butterfly splitfin Ameca splendens. Folia Biologica (Kraków) 67: 53-60. The butterfly splitfin (Ameca splendens) is a fish species that belongs to the Goodeidae family. The biology of this species, which is today at risk of extinction in its natural habitats, has not been fully explored. The objective of the present study was to characterize melanomacrophages (MMs) and the melanomacrophage centers (MMCs) that they form, associated with the immunological system of butterfly splitfin. Butterfly splitfin is a potential new model species with a placenta for scientific research. In addition, knowledge about the location and characteristics of MMCs will allow the effective development of a conservation strategy for this species and monitoring of the natural environment. The results of histological analyses show that the immune system of fish aged 1 dph was completely developed. Single MMs were observed in hepatic sinusoids and in head kidney and their agglomerations were noticed in the exocrine pancreas and in the spleen. Hemosiderin was detected in single MMs in the head kidney of fish aged 1 dph, and in the spleen, exocrine pancreas and liver of older fish. -

AC Summer 2008
9 American Currents Vol. 34, No. 3 The Mummichog: Master of Survival Robert Bock 1602 Tilton Dr., Silver Spring, MD 20902, [email protected] Photographs by David Snell ew aquarium hobbyists have even heard of the filled an old laundry sink with a few inches of water, straight Mummichog. Others know it only as a bait fish. from the tap, and dropped them in. Chlorine didn’t appear to Yet this determined survivor is not only easy to care bother them too much. They lived for months. F for, but an interesting and attractive species worthy Back then, New Jersey’s Hackensack meadowlands were of aquarium study. shamefully polluted, both from massive garbage dumps that The Mummichog, Fundulus heteroclitus, occurs in the filled the marshes and the many factories that sprang up along tidal waters of North America, from the Gulf of Saint the river. I can remember taking a few steps along the river Lawrence southward to northeastern Florida. The name bank, then looking back and seeing heavy black oil oozing “Mummichog” comes from an American Indian word that from my footprints. means “they go in great numbers.” Reaching a maximum Yet Mummichog were abundant. Frequently, I saw shoals length of about five inches, Mummichog are a shoaling fish of at least a thousand in the shallows. Similarly, shoals of that prefer the quieter waters of estuaries and salt marshes. Mummichog covered the surface of the toxin-laden ponds Like many coastal species, they can tolerate a wide range of that formed at the base of the landfills. salinities, ranging from fresh water to sea water. -

Copyrighted Material
Index INDEX Note: page numbers in italics refer to fi gures, those in bold refer to tables and boxes. abducens nerve 55 activity cycles 499–522 inhibition 485 absorption effi ciency 72 annual patterns 515, 516, 517–22 interactions 485–6 abyssal zone 393 circadian rhythms 505 prey 445 Acanthaster planci (Crown-of-Thorns Starfi sh) diel patterns 499, 500, 501–2, 503–4, reduction 484 579 504–7 aggressive mimicry 428, 432–3 Acanthocybium (Wahoo) 15 light-induced 499, 500, 501–2, 503–4, aggressive resemblance 425–6 Acanthodii 178, 179 505 aglomerular 52 Acanthomorpha 284–8, 289 lunar patterns 507–9 agnathans Acanthopterygii 291–325 seasonal 509–15 gills 59, 60 Atherinomorpha 293–6 semilunar patterns 507–9 osmoregulation 101, 102 characteristics 291–2 supra-annual patterns 515, 516, 517–22 phylogeny 202 distribution 349, 350 tidal patterns 506–7 ventilation 59, 60 jaws 291 see also migration see also hagfi shes; lampreys Mugilomorpha 292–3, 294 adaptive response 106 agnathous fi shes see jawless fi shes pelagic 405 adaptive zones 534 agonistic interactions 83–4, 485–8 Percomorpha 296–325 adenohypophysis 91, 92 chemically mediated 484 pharyngeal jaws 291 adenosine triphosphate (ATP) 57 sound production 461–2 phylogeny 292, 293, 294 adipose fi n 35 visual 479 spines 449, 450 adrenocorticotropic hormone (ACTH) 92 agricultural chemicals 605 Acanthothoraciformes 177 adrianichthyids 295 air breathing 60, 61–2, 62–4 acanthurids 318–19 adult fi shes 153, 154, 155–7 ammonia production 64, 100–1 Acanthuroidei 12, 318–19 death 156–7 amphibious 60 Acanthurus bahianus -
Teleostei, Syngnathidae)
ZooKeys 934: 141–156 (2020) A peer-reviewed open-access journal doi: 10.3897/zookeys.934.50924 RESEARCH ARTICLE https://zookeys.pensoft.net Launched to accelerate biodiversity research Hippocampus nalu, a new species of pygmy seahorse from South Africa, and the first record of a pygmy seahorse from the Indian Ocean (Teleostei, Syngnathidae) Graham Short1,2,3, Louw Claassens4,5,6, Richard Smith4, Maarten De Brauwer7, Healy Hamilton4,8, Michael Stat9, David Harasti4,10 1 Research Associate, Ichthyology, Australian Museum Research Institute, Sydney, Australia 2 Ichthyology, California Academy of Sciences, San Francisco, USA 3 Ichthyology, Burke Museum, Seattle, USA 4 IUCN Seahorse, Pipefish Stickleback Specialist Group, University of British Columbia, Vancouver, Canada5 Rhodes University, Grahamstown, South Africa 6 Knysna Basin Project, Knysna, South Africa 7 University of Leeds, Leeds, UK 8 NatureServe, Arlington, Virginia, USA 9 University of Newcastle, Callaghan, NSW, Australia 10 Port Stephens Fisheries Institute, NSW, Australia Corresponding author: Graham Short ([email protected]) Academic editor: Nina Bogutskaya | Received 13 February 2020 | Accepted 12 April 2020 | Published 19 May 2020 http://zoobank.org/E9104D84-BB71-4533-BB7A-2DB3BD4E4B5E Citation: Short G, Claassens L, Smith R, De Brauwer M, Hamilton H, Stat M, Harasti D (2020) Hippocampus nalu, a new species of pygmy seahorse from South Africa, and the first record of a pygmy seahorse from the Indian Ocean (Teleostei, Syngnathidae). ZooKeys 934: 141–156. https://doi.org/10.3897/zookeys.934.50924 Abstract A new species and the first confirmed record of a true pygmy seahorse from Africa,Hippocampus nalu sp. nov., is herein described on the basis of two specimens, 18.9–22 mm SL, collected from flat sandy coral reef at 14–17 meters depth from Sodwana Bay, South Africa. -

Atividade De Limpeza E Clientes De Elacatinus Figaro (Pisces: Gobiidae) Nos Recifes De Coral Dos Parrachos De Muriú, Nordeste Do Brasil
Atividade de limpeza e clientes de Elacatinus figaro (Pisces: Gobiidae) nos recifes de coral dos Parrachos de Muriú, Nordeste do Brasil Campos, C.E.C. & Sá-Oliveira, J.C. Biota Neotrop. 2011, 11(1): 47-52. On line version of this paper is available from: http://www.biotaneotropica.org.br/v11n1/en/abstract?article+bn01011012011 A versão on-line completa deste artigo está disponível em: http://www.biotaneotropica.org.br/v11n1/pt/abstract?article+bn01011012011 Received/ Recebido em 30/06/2010 - Revised/ Versão reformulada recebida em 14/12/2010 - Accepted/ Publicado em 11/01/2011 ISSN 1676-0603 (on-line) Biota Neotropica is an electronic, peer-reviewed journal edited by the Program BIOTA/FAPESP: The Virtual Institute of Biodiversity. This journal’s aim is to disseminate the results of original research work, associated or not to the program, concerned with characterization, conservation and sustainable use of biodiversity within the Neotropical region. Biota Neotropica é uma revista do Programa BIOTA/FAPESP - O Instituto Virtual da Biodiversidade, que publica resultados de pesquisa original, vinculada ou não ao programa, que abordem a temática caracterização, conservação e uso sustentável da biodiversidade na região Neotropical. Biota Neotropica is an eletronic journal which is available free at the following site http://www.biotaneotropica.org.br A Biota Neotropica é uma revista eletrônica e está integral e gratuitamente disponível no endereço http://www.biotaneotropica.org.br Biota Neotrop., vol. 11, no. 1 Atividade de limpeza e clientes de Elacatinus figaro (Pisces: Gobiidae) nos recifes de coral dos Parrachos de Muriú, Nordeste do Brasil Carlos Eduardo Costa Campos1,2 & Júlio César Sá-Oliveira1 1 Laboratório de Zoologia, Departamento de Ciências Biológicas, Universidade Federal do Amapá – UNIFAP, Rod. -

Volume 19 Winter 2002 the Coral Hind, Lapu Lapu, Or Miniata
FREE ISSN 1045-3520 Volume 19 Winter 2002 Introducing a Zonal Based Natural Photo by Robert Fenner Filtration System for Reef Aquariums by Steve Tyree Quite a few natural based filtration systems have been devised by reef aquarists and scientists in the past twenty years. Some systems utilized algae to remove organic and inorganic pollutants from the reef aquarium; others utilized sediment beds. The natural filtration system that I have been researching and designing is drastically different from both of these types. No external algae are used. I believe that all the algae a functional reef requires are already growing in the reef, even if they are not apparent. They include micro-algae, turf algae, coralline algae, single-cell algae within photosynthetic corals, and cyanobacteria with photosynthetic capabilities. Most of the systems that I have set up to research this concept have not included sediment beds. All organic matter and pollutants are recycled and processed within the system by macro-organisms. Sediment beds have not been utilized to process excess Miniata Grouper, Cephalopholis miniata organic debris, but that does not prevent other aquarists from adding them. The main concept behind my system is the use of living sponges, sea squirts, and filter feeders for filtration. Sponges consume bacteria, can reach about twenty inches in length in the wild, and dissolved and colloidal organic material, micro-plankton, The Coral Hind, Lapu about half that in captivity. It is undoubtedly the most and fine particulate matter. Sea squirts consume large Lapu, or Miniata prized member of the genus for the aquarium trade. -

Identification and Modelling of a Representative Vulnerable Fish Species for Pesticide Risk Assessment in Europe
Identification and Modelling of a Representative Vulnerable Fish Species for Pesticide Risk Assessment in Europe Von der Fakultät für Mathematik, Informatik und Naturwissenschaften der RWTH Aachen University zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften genehmigte Dissertation vorgelegt von Lara Ibrahim, M.Sc. aus Mazeraat Assaf, Libanon Berichter: Universitätsprofessor Dr. Andreas Schäffer Prof. Dr. Christoph Schäfers Tag der mündlichen Prüfung: 30. Juli 2015 Diese Dissertation ist auf den Internetseiten der Universitätsbibliothek online verfügbar Erklärung Ich versichere, dass ich diese Doktorarbeit selbständig und nur unter Verwendung der angegebenen Hilfsmittel angefertigt habe. Weiterhin versichere ich, die aus benutzten Quellen wörtlich oder inhaltlich entnommenen Stellen als solche kenntlich gemacht zu haben. Lara Ibrahim Aachen, am 18 März 2015 Zusammenfassung Die Zulassung von Pflanzenschutzmitteln in der Europäischen Gemeinschaft verlangt unter anderem eine Abschätzung des Risikos für Organismen in der Umwelt, die nicht Ziel der Anwendung sind. Unvertretbare Auswirkungen auf den Naturhalt sollen vermieden werden. Die ökologische Risikoanalyse stellt die dafür benötigten Informationen durch eine Abschätzung der Exposition der Organismen und der sich daraus ergebenden Effekte bereit. Die Effektabschätzung beruht dabei hauptsächlich auf standardisierten ökotoxikologischen Tests im Labor mit wenigen, oft nicht einheimischen Stellvertreterarten. In diesen Tests werden z. B. Effekte auf das Überleben, das Wachstum und/oder die Reproduktion von Fischen bei verschiedenen Konzentrationen der Testsubstanz gemessen und Endpunkte wie die LC50 (Lethal Concentrations for 50%) oder eine NOEC (No Observed Effect Concentration, z. B. für Wachstum oder Reproduktionsparameter) abgeleitet. Für Fische und Wirbeltiere im Allgemeinen beziehen sich die spezifischen Schutzziele auf das Überleben von Individuen und die Abundanz und Biomasse von Populationen. -

Venom Evolution Widespread in Fishes: a Phylogenetic Road Map for the Bioprospecting of Piscine Venoms
Journal of Heredity 2006:97(3):206–217 ª The American Genetic Association. 2006. All rights reserved. doi:10.1093/jhered/esj034 For permissions, please email: [email protected]. Advance Access publication June 1, 2006 Venom Evolution Widespread in Fishes: A Phylogenetic Road Map for the Bioprospecting of Piscine Venoms WILLIAM LEO SMITH AND WARD C. WHEELER From the Department of Ecology, Evolution, and Environmental Biology, Columbia University, 1200 Amsterdam Avenue, New York, NY 10027 (Leo Smith); Division of Vertebrate Zoology (Ichthyology), American Museum of Natural History, Central Park West at 79th Street, New York, NY 10024-5192 (Leo Smith); and Division of Invertebrate Zoology, American Museum of Natural History, Central Park West at 79th Street, New York, NY 10024-5192 (Wheeler). Address correspondence to W. L. Smith at the address above, or e-mail: [email protected]. Abstract Knowledge of evolutionary relationships or phylogeny allows for effective predictions about the unstudied characteristics of species. These include the presence and biological activity of an organism’s venoms. To date, most venom bioprospecting has focused on snakes, resulting in six stroke and cancer treatment drugs that are nearing U.S. Food and Drug Administration review. Fishes, however, with thousands of venoms, represent an untapped resource of natural products. The first step in- volved in the efficient bioprospecting of these compounds is a phylogeny of venomous fishes. Here, we show the results of such an analysis and provide the first explicit suborder-level phylogeny for spiny-rayed fishes. The results, based on ;1.1 million aligned base pairs, suggest that, in contrast to previous estimates of 200 venomous fishes, .1,200 fishes in 12 clades should be presumed venomous. -
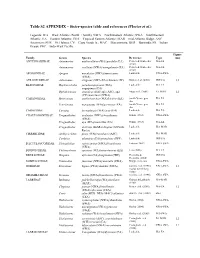
Table S2 APPENDIX – Sister-Species Table and References (Floeter Et Al.)
Table S2 APPENDIX – Sister-species table and references (Floeter et al.) Legends: WA = West Atlantic (North + South); NWA = Northwestern Atlantic; SWA = Southwestern Atlantic; EA = Eastern Atlantic; TEA = Tropical Eastern Atlantic; MAR = mid-Atlantic Ridge; ASC = Ascension; SHE = St. Helena; CV = Cape Verde Is.; MAC = Macaronesia; BER = Bermuda; IO = Indian Ocean; IWP = Indo-West Pacific. Figure Family Genus Species Reference Type (ms) ANTENNARIIDAE Antennarius multiocellatus (WA)/pardalis (EA) Pietsch & Grobecker WA-EA (1987) Antennarius ocellatus (NWA)/senegalensis (EA) Pietsch & Grobecker WA-EA (1987) APOGONIDAE Apogon maculatus (NWA)/americanus Look alike NWA-SWA (SWA) AULOSTOMIDAE Aulostomus strigosus (SWA-EA)/chinensis (IP) Bowen et al. (2001) IWP-EA 12 BLENNIIDAE Hypleurochilus pseudoaequipinnis (WA)/ Look alike WA-EA aequipinnis (EA) Ophioblennius atlanticus (EA)/ sp1 (ASC), sp2 Muss et al. (2001) EA-MAR 12 (CV)/macclurei (NWA) CARANGIDAE Hemicaranx amblyrhynchus (WA)/bicolor (EA) Smith-Vaniz, pers WA-EA obs. Trachinotus marginatus (WA)/goreensis (EA) Smith-Vaniz, pers WA-EA obs. CARAPIDAE Carapus bermudensis (WA)/acus (EA) Look alike WA-EA CHAETODONTIDAE Prognathodes aculeatus (NWA)/brasiliensis Hubbs (1963) NWA-SWA (SWA) Prognathodes aya (WA)/marcellae (EA) Hubbs (1963) WA-EA Prognathodes dichrous (MAR)/obliquus (St Paul's Look alike WA-MAR Rocks) CIRRHITIDAE Amblycirrhitus pinos (WA)/earnshawi (ASC) Look alike WA-MAR Cirrhitus atlanticus (EA)/pinnulatus (IWP) Look alike IWP-EA DACTYLOSCOPIDAE Platygillellus rubrocinctus (NWA)/brasiliensis Feitoza (2002) NWA-SWA (SWA) DIODONTIDAE Chilomycterus spinosus (WA)/mauretanicus (EA) Leis (2006) WA-EA DREPANIDAE Drepane africana (EA)/longimana (IWP) Heemstra & IWP-EA Heemstra (2004) GOBIESOCIDAE Tomicodon fasciatus (NWA)/australis (SWA) Briggs, pers com NWA-SWA GOBIIDAE Elacatinus figaro (SWA)/randalli (NWA) Sazima et al. -

Download Fishlore.Com's Saltwater Aquarium and Reef Tank E-Book
Updated: August 6, 2013 This e-Book is FREE for public use. Commercial use prohibited. Copyright FishLore.com – providing tropical fish tank and aquarium fish information for freshwater fish and saltwater fish keepers. FishLore.com Saltwater Aquarium & Reef Tank e-Book 1 CONTENTS Foreword .......................................................................................................................................... 10 Why Set Up an Aquarium? .............................................................................................................. 12 Aquarium Types ............................................................................................................................... 14 Aquarium Electrical Safety ............................................................................................................... 15 Aquarium Fish Cruelty Through Ignorance ..................................................................................... 17 The Aquarium Nitrogen Cycle ......................................................................................................... 19 Aquarium Filter and Fish Tank Filtration ......................................................................................... 24 Saltwater Aquarium Types - FOWLR, Fish Only with Live Rock, Reef Tank .................................... 30 Freshwater Aquarium vs. Saltwater Aquarium ............................................................................... 33 Saltwater Aquarium Tank Setup Guide .......................................................................................... -

The Global Trade in Marine Ornamental Species
From Ocean to Aquarium The global trade in marine ornamental species Colette Wabnitz, Michelle Taylor, Edmund Green and Tries Razak From Ocean to Aquarium The global trade in marine ornamental species Colette Wabnitz, Michelle Taylor, Edmund Green and Tries Razak ACKNOWLEDGEMENTS UNEP World Conservation This report would not have been The authors would like to thank Helen Monitoring Centre possible without the participation of Corrigan for her help with the analyses 219 Huntingdon Road many colleagues from the Marine of CITES data, and Sarah Ferriss for Cambridge CB3 0DL, UK Aquarium Council, particularly assisting in assembling information Tel: +44 (0) 1223 277314 Aquilino A. Alvarez, Paul Holthus and and analysing Annex D and GMAD data Fax: +44 (0) 1223 277136 Peter Scott, and all trading companies on Hippocampus spp. We are grateful E-mail: [email protected] who made data available to us for to Neville Ash for reviewing and editing Website: www.unep-wcmc.org inclusion into GMAD. The kind earlier versions of the manuscript. Director: Mark Collins assistance of Akbar, John Brandt, Thanks also for additional John Caldwell, Lucy Conway, Emily comments to Katharina Fabricius, THE UNEP WORLD CONSERVATION Corcoran, Keith Davenport, John Daphné Fautin, Bert Hoeksema, Caroline MONITORING CENTRE is the biodiversity Dawes, MM Faugère et Gavand, Cédric Raymakers and Charles Veron; for assessment and policy implemen- Genevois, Thomas Jung, Peter Karn, providing reprints, to Alan Friedlander, tation arm of the United Nations Firoze Nathani, Manfred Menzel, Julie Hawkins, Sherry Larkin and Tom Environment Programme (UNEP), the Davide di Mohtarami, Edward Molou, Ogawa; and for providing the picture on world’s foremost intergovernmental environmental organization. -

A Reappraisal of Stegastes Species Occurring in the South Atlantic Using
de Souza et al. Helgol Mar Res (2016) 70:20 DOI 10.1186/s10152-016-0471-x Helgoland Marine Research ORIGINAL ARTICLE Open Access A reappraisal of Stegastes species occurring in the South Atlantic using morphological and molecular data Allyson Santos de Souza1*, Ricardo de Souza Rosa2, Rodrigo Xavier Soares1, Paulo Augusto de Lima‑Filho3, Claudio de Oliveira4, Oscar Akio Shibatta5 and Wagner Franco Molina1 Abstract The taxonomic status of Pomacentridae species can be difficult to determine, due to the high diversity, and in some cases, poorly understood characters, such as color patterns. Although Stegastes rocasensis, endemic to the Rocas atoll and Fernando de Noronha archipelago, and S. sanctipauli, endemic to the São Pedro and São Paulo archipelago, differ in color pattern, they exhibit similar morphological characters and largely overlapping counts of fin rays and lateral-line scales. Another nominal insular species, S. trindadensis, has recently been synonymized with S. fuscus but retained as a valid subspecies by some authors. Counts and morphometric analyses and mitochondrial DNA (COI, 16SrRNA, CytB) and nuclear DNA (rag1 and rhodopsin) comparisons of three insular species (S. rocasensis, S. sanctipauli and S. trindadensis) and three other South Atlantic species (S. fuscus, S. variabilis and S. pictus) were carried out in the present study. Analyses of the principal components obtained by traditional multivariate morphometry indicate that the species in general have similar body morphology. Molecular analyses revealed conspicuous similarity between S. rocasensis and S. sanctipauli and between S. trindadensis and S. fuscus and a clear divergence between S. variabilis from Northeast Brazil and S. variabilis from the Caribbean region.