Gobiiformes: Gobiidae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Article Evolutionary Dynamics of the OR Gene Repertoire in Teleost Fishes
bioRxiv preprint doi: https://doi.org/10.1101/2021.03.09.434524; this version posted March 10, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Article Evolutionary dynamics of the OR gene repertoire in teleost fishes: evidence of an association with changes in olfactory epithelium shape Maxime Policarpo1, Katherine E Bemis2, James C Tyler3, Cushla J Metcalfe4, Patrick Laurenti5, Jean-Christophe Sandoz1, Sylvie Rétaux6 and Didier Casane*,1,7 1 Université Paris-Saclay, CNRS, IRD, UMR Évolution, Génomes, Comportement et Écologie, 91198, Gif-sur-Yvette, France. 2 NOAA National Systematics Laboratory, National Museum of Natural History, Smithsonian Institution, Washington, D.C. 20560, U.S.A. 3Department of Paleobiology, National Museum of Natural History, Smithsonian Institution, Washington, D.C., 20560, U.S.A. 4 Independent Researcher, PO Box 21, Nambour QLD 4560, Australia. 5 Université de Paris, Laboratoire Interdisciplinaire des Energies de Demain, Paris, France 6 Université Paris-Saclay, CNRS, Institut des Neurosciences Paris-Saclay, 91190, Gif-sur- Yvette, France. 7 Université de Paris, UFR Sciences du Vivant, F-75013 Paris, France. * Corresponding author: e-mail: [email protected]. !1 bioRxiv preprint doi: https://doi.org/10.1101/2021.03.09.434524; this version posted March 10, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Teleost fishes perceive their environment through a range of sensory modalities, among which olfaction often plays an important role. -

Bancodosabrolhos Cad
1 Banco dos Abrolhos & Cadeia Vitória-Trindade 3 Proposta de reconhecimento de uma Reserva da Biosfera Marinha na Costa Central do Brasil Banco dos Abrolhos & Cadeia Vitória-Trindade Proposta de reconhecimento de uma Reserva da Biosfera Marinha na Costa Central do Brasil 3 AGRADECIMENTOS EQUIPE RBMA Comites Estaduais da RBMA na Bahia e Espírito Santo Secretário Executivo : Luiz Alberto Bucci Colegiado Mar da RBMA Apoio técnico : Grupo Conexão Abrolhos-Trindade Ana Lopez Bahia, Espírito Santo e Rio de Janeiro Heloisa Dias Marcelo M. Amaral Postos Avançados da RBMA Nilson Máximo Parque Nacional Marinho de Abrolhos Pedro Castro Base TAMAR-Linhares Apoio Administrativo: Secretaria de Biodiversidade e Fernando Capello Floresta / MMA Luan Vasco Leiz da Silva Rosa UNESCO - Oficina Montevideo Oswaldo Henrique de Souza Fotos e Mapas: Editoração Grafica: Arquivos Voz da Natureza & RBMA Felipe Sleiman Sumário APRESENTAÇÃO .................................................................................... 06 I47b INSTITUTO AMIGOS DA RESERVA DA BIOSFERA DA MATA ATLÂNTICA 1. UMA RESERVA DA BIOSFERA MARINHA NO BRASIL ............ 08 Banco dos Abrolhos & Cadeia Vitoria-Trindade: Proposta de reconhecimento de uma Reserva da Biosfera marinha na Costa Central do Brasil. 2. ECOSSISTEMAS E AMBIENTES DA REGIÃO .............................. 12 Organização Clayton Ferreira Lino; Heloisa Dias São Paulo: IA-RBMA, 2014 63p. ; il. 27 cm 3. ASPECTOS DA BIODIVERSIDADE ............................................... 18 Disponível também em: http://www.rbma.org.br 4. SERVIÇOS ECOSSISTÊMICOS ........................................................ 30 Bibliografia 5. PRINCIPAIS AMEAÇAS A BIODIVERSIDADE ............................. 34 ISBN: 978-85-68863-00-8 1. Reserva Biosfera Marinha-Brasil 2. Ecossistemas-ambientais 6. ÁREAS PROTEGIDAS ........................................................................ 42 3. Biodiversidade Marinha 4. Áreas protegidas. 5. Sustentabilidade I. Lino Clayton Ferreira, org. II. Mazzei Eric Freitas, org.III. -

Assessing Species Diversity of Coral Triangle Artisanal Fisheries: a DNA Barcode Reference Library for the Shore Fishes Retailed at Ambon Harbor (Indonesia)
Received: 19 September 2019 | Revised: 30 January 2020 | Accepted: 3 February 2020 DOI: 10.1002/ece3.6128 ORIGINAL RESEARCH Assessing species diversity of Coral Triangle artisanal fisheries: A DNA barcode reference library for the shore fishes retailed at Ambon harbor (Indonesia) Gino Limmon1 | Erwan Delrieu-Trottin2,3 | Jesaya Patikawa1 | Frederik Rijoly1 | Hadi Dahruddin4 | Frédéric Busson2,5 | Dirk Steinke6 | Nicolas Hubert2 1Pusat Kemaritiman dan Kelautan, Universitas Pattimura (Maritime and Marine Abstract Science Center of Excellence), Ambon, The Coral Triangle (CT), a region spanning across Indonesia and Philippines, is home Indonesia to about 4,350 marine fish species and is among the world's most emblematic re- 2Institut de Recherche pour le Développement, UMR 226 ISEM (UM- gions in terms of conservation. Threatened by overfishing and oceans warming, the CNRS-IRD-EPHE), Montpellier, France CT fisheries have faced drastic declines over the last decades. Usually monitored 3Museum für Naturkunde, Leibniz-Institut für Evolutions-und Biodiversitätsforschung through a biomass-based approach, fisheries trends have rarely been characterized an der Humboldt-Universität zu Berlin, at the species level due to the high number of taxa involved and the difficulty to Berlin, Germany accurately and routinely identify individuals to the species level. Biomass, however, 4Division of Zoology, Research Center for Biology, Indonesian Institute of Sciences is a poor proxy of species richness, and automated methods of species identifica- (LIPI), Cibinong, Indonesia tion are required to move beyond biomass-based approaches. Recent meta-analyses 5UMR 7208 BOREA (MNHN-CNRS-UPMC- have demonstrated that species richness peaks at intermediary levels of biomass. IRD-UCBN), Muséum National d’Histoire Naturelle, Paris, France Consequently, preserving biomass is not equal to preserving biodiversity. -

Atividade De Limpeza E Clientes De Elacatinus Figaro (Pisces: Gobiidae) Nos Recifes De Coral Dos Parrachos De Muriú, Nordeste Do Brasil
Atividade de limpeza e clientes de Elacatinus figaro (Pisces: Gobiidae) nos recifes de coral dos Parrachos de Muriú, Nordeste do Brasil Campos, C.E.C. & Sá-Oliveira, J.C. Biota Neotrop. 2011, 11(1): 47-52. On line version of this paper is available from: http://www.biotaneotropica.org.br/v11n1/en/abstract?article+bn01011012011 A versão on-line completa deste artigo está disponível em: http://www.biotaneotropica.org.br/v11n1/pt/abstract?article+bn01011012011 Received/ Recebido em 30/06/2010 - Revised/ Versão reformulada recebida em 14/12/2010 - Accepted/ Publicado em 11/01/2011 ISSN 1676-0603 (on-line) Biota Neotropica is an electronic, peer-reviewed journal edited by the Program BIOTA/FAPESP: The Virtual Institute of Biodiversity. This journal’s aim is to disseminate the results of original research work, associated or not to the program, concerned with characterization, conservation and sustainable use of biodiversity within the Neotropical region. Biota Neotropica é uma revista do Programa BIOTA/FAPESP - O Instituto Virtual da Biodiversidade, que publica resultados de pesquisa original, vinculada ou não ao programa, que abordem a temática caracterização, conservação e uso sustentável da biodiversidade na região Neotropical. Biota Neotropica is an eletronic journal which is available free at the following site http://www.biotaneotropica.org.br A Biota Neotropica é uma revista eletrônica e está integral e gratuitamente disponível no endereço http://www.biotaneotropica.org.br Biota Neotrop., vol. 11, no. 1 Atividade de limpeza e clientes de Elacatinus figaro (Pisces: Gobiidae) nos recifes de coral dos Parrachos de Muriú, Nordeste do Brasil Carlos Eduardo Costa Campos1,2 & Júlio César Sá-Oliveira1 1 Laboratório de Zoologia, Departamento de Ciências Biológicas, Universidade Federal do Amapá – UNIFAP, Rod. -

Co-Evolution of Cleaning and Feeding Morphology in Western Atlantic and Eastern Pacific Gobies
ORIGINAL ARTICLE doi:10.1111/evo.13904 Co-evolution of cleaning and feeding morphology in western Atlantic and eastern Pacific gobies Jonathan M. Huie,1,2 Christine E. Thacker,3,4 and Luke Tornabene1,5 1School of Aquatic and Fishery Sciences, University of Washington, 1122 NE Boat St, Seattle, Washington 98195 2E-mail: [email protected] 3Santa Barbara Museum of Natural History, 2559 Puesta del Sol, Santa Barbara, California 93105 4Natural History Museum of Los Angeles County, 900 Exposition Blvd, Los Angeles, California 90007 5Burke Museum of Natural History and Culture, 4300 15th Ave NE, Seattle, Washington 98105 Received September 2, 2019 Accepted November 25, 2019 Cleaning symbioses are mutualistic relationships where cleaners remove and consume ectoparasites from their clients. Cleaning behavior is rare in fishes and is a highly specialized feeding strategy only observed in around 200 species. Cleaner fishes vary in their degree of specialization, ranging from species that clean as juveniles or facultatively as adults, to nearly obligate or dedicated cleaners. Here, we investigate whether these different levels of trophic specialization correspond with similar changes in feeding morphology. Specifically, we model the evolution of cleaning behavior across the family Gobiidae, which contains the most speciose radiation of dedicated and facultative cleaner fishes. We compared the cranial morphology and dentition of cleaners and non-cleaners across the phylogeny of cleaning gobies and found that facultative cleaners independently evolved four times and have converged on an intermediate morphology relative to that of dedicated cleaners and non-cleaning generalists. This is consistent with their more flexible feeding habits. Cleaner gobies also possess a distinct tooth morphology, which suggests they are adapted for scraping parasites off their clients and show little similarity to other cleaner clades. -
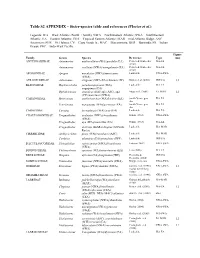
Table S2 APPENDIX – Sister-Species Table and References (Floeter Et Al.)
Table S2 APPENDIX – Sister-species table and references (Floeter et al.) Legends: WA = West Atlantic (North + South); NWA = Northwestern Atlantic; SWA = Southwestern Atlantic; EA = Eastern Atlantic; TEA = Tropical Eastern Atlantic; MAR = mid-Atlantic Ridge; ASC = Ascension; SHE = St. Helena; CV = Cape Verde Is.; MAC = Macaronesia; BER = Bermuda; IO = Indian Ocean; IWP = Indo-West Pacific. Figure Family Genus Species Reference Type (ms) ANTENNARIIDAE Antennarius multiocellatus (WA)/pardalis (EA) Pietsch & Grobecker WA-EA (1987) Antennarius ocellatus (NWA)/senegalensis (EA) Pietsch & Grobecker WA-EA (1987) APOGONIDAE Apogon maculatus (NWA)/americanus Look alike NWA-SWA (SWA) AULOSTOMIDAE Aulostomus strigosus (SWA-EA)/chinensis (IP) Bowen et al. (2001) IWP-EA 12 BLENNIIDAE Hypleurochilus pseudoaequipinnis (WA)/ Look alike WA-EA aequipinnis (EA) Ophioblennius atlanticus (EA)/ sp1 (ASC), sp2 Muss et al. (2001) EA-MAR 12 (CV)/macclurei (NWA) CARANGIDAE Hemicaranx amblyrhynchus (WA)/bicolor (EA) Smith-Vaniz, pers WA-EA obs. Trachinotus marginatus (WA)/goreensis (EA) Smith-Vaniz, pers WA-EA obs. CARAPIDAE Carapus bermudensis (WA)/acus (EA) Look alike WA-EA CHAETODONTIDAE Prognathodes aculeatus (NWA)/brasiliensis Hubbs (1963) NWA-SWA (SWA) Prognathodes aya (WA)/marcellae (EA) Hubbs (1963) WA-EA Prognathodes dichrous (MAR)/obliquus (St Paul's Look alike WA-MAR Rocks) CIRRHITIDAE Amblycirrhitus pinos (WA)/earnshawi (ASC) Look alike WA-MAR Cirrhitus atlanticus (EA)/pinnulatus (IWP) Look alike IWP-EA DACTYLOSCOPIDAE Platygillellus rubrocinctus (NWA)/brasiliensis Feitoza (2002) NWA-SWA (SWA) DIODONTIDAE Chilomycterus spinosus (WA)/mauretanicus (EA) Leis (2006) WA-EA DREPANIDAE Drepane africana (EA)/longimana (IWP) Heemstra & IWP-EA Heemstra (2004) GOBIESOCIDAE Tomicodon fasciatus (NWA)/australis (SWA) Briggs, pers com NWA-SWA GOBIIDAE Elacatinus figaro (SWA)/randalli (NWA) Sazima et al. -

Patterns of Evolution in Gobies (Teleostei: Gobiidae): a Multi-Scale Phylogenetic Investigation
PATTERNS OF EVOLUTION IN GOBIES (TELEOSTEI: GOBIIDAE): A MULTI-SCALE PHYLOGENETIC INVESTIGATION A Dissertation by LUKE MICHAEL TORNABENE BS, Hofstra University, 2007 MS, Texas A&M University-Corpus Christi, 2010 Submitted in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY in MARINE BIOLOGY Texas A&M University-Corpus Christi Corpus Christi, Texas December 2014 © Luke Michael Tornabene All Rights Reserved December 2014 PATTERNS OF EVOLUTION IN GOBIES (TELEOSTEI: GOBIIDAE): A MULTI-SCALE PHYLOGENETIC INVESTIGATION A Dissertation by LUKE MICHAEL TORNABENE This dissertation meets the standards for scope and quality of Texas A&M University-Corpus Christi and is hereby approved. Frank L. Pezold, PhD Chris Bird, PhD Chair Committee Member Kevin W. Conway, PhD James D. Hogan, PhD Committee Member Committee Member Lea-Der Chen, PhD Graduate Faculty Representative December 2014 ABSTRACT The family of fishes commonly known as gobies (Teleostei: Gobiidae) is one of the most diverse lineages of vertebrates in the world. With more than 1700 species of gobies spread among more than 200 genera, gobies are the most species-rich family of marine fishes. Gobies can be found in nearly every aquatic habitat on earth, and are often the most diverse and numerically abundant fishes in tropical and subtropical habitats, especially coral reefs. Their remarkable taxonomic, morphological and ecological diversity make them an ideal model group for studying the processes driving taxonomic and phenotypic diversification in aquatic vertebrates. Unfortunately the phylogenetic relationships of many groups of gobies are poorly resolved, obscuring our understanding of the evolution of their ecological diversity. This dissertation is a multi-scale phylogenetic study that aims to clarify phylogenetic relationships across the Gobiidae and demonstrate the utility of this family for studies of macroevolution and speciation at multiple evolutionary timescales. -

Status of Gobiosoma (Teleostei: Gobiidae) from Brazil: Description of a New Species, Redescription of G
Zootaxa 4007 (4): 451–480 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2015 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.4007.4.1 http://zoobank.org/urn:lsid:zoobank.org:pub:F6A8C8AF-5A2B-4F07-9679-5F0E026E776F Status of Gobiosoma (Teleostei: Gobiidae) from Brazil: description of a new species, redescription of G. hemigymnum, molecular phylogeny of the genus, and key to Atlantic species JAMES L. VAN TASSELL1, JEAN-CHRISTOPHE JOYEUX2, RAPHAEL MARIANO MACIEIRA2 & LUKE TORNABENE3 1American Museum of Natural History, New York, USA. E-mail: [email protected] 2Universidade Federal do Espírito Santo, Brazil. E-mail: [email protected], [email protected] 3Texas A&M University - Corpus Christi, Texas, USA. E-mail: [email protected] Abstract It is unclear how many species of Gobiosoma occur in Brazil and what their geographic distributions are. Here we combine data from a comprehensive morphological survey and a molecular analysis to clarify this uncertain taxonomy and place Brazilian Gobiosoma within a phylogenetic framework. Recent collections in Brazil, from the states of Ceará to Santa Catarina, and in Uruguay yielded two allopatric species of Gobiosoma that are distinct in genetics, meristics, morphomet- rics, scale pattern and coloration. Comparisons were made with types and specimens of Gobiosoma hemigymnum, Gar- mannia mediocricula, Gobiosoma spilotum and Gobiosoma parri and all other known species of Gobiosoma. We place G. parri in synonomy with G. hemigymnum with a distribution of Rio de Janeiro to Uruguay and Argentina. The northern species, that extends from the states of Espírito Santo to Ceará, is described as a new species, Gobiosoma alfiei. -

Development of Larval Fish Rearing Techniques and Nutrient Requirements for the Green Mandarin, Synchiropus Splendidus: a Popular Marine Ornamental Fish
ResearchOnline@JCU This file is part of the following reference: Shao, Luchang (2016) Development of larval fish rearing techniques and nutrient requirements for the green mandarin, Synchiropus splendidus: a popular marine ornamental fish. PhD thesis, James Cook University. Access to this file is available from: http://researchonline.jcu.edu.au/47308/ The author has certified to JCU that they have made a reasonable effort to gain permission and acknowledge the owner of any third party copyright material included in this document. If you believe that this is not the case, please contact [email protected] and quote http://researchonline.jcu.edu.au/47308/ Development of larval fish rearing techniques and nutrient requirement for the green mandarin, Synchiropus splendidus: a popular marine ornamental fish Thesis submitted by Luchang Shao (MSc) in September 2016 For the degree of Doctor of Philosophy In the College of Marine and Environmental Science James Cook University Declaration on Ethics The research presented and reported in this thesis was conducted within the guidelines for research ethics outlined in the National Statement on Ethics Conduct in Research Involving Human (1999), the Joint NHMRC/AVCC Statement and Guidelines on Research Practice (1997), the James Cook University Policy on Experimentation Ethics Standard Practices and Guidelines (2001), and the James Cook University Statement and Guidelines on Research Practice (2001). The proposed research methodology received clearance from the James Cook University Experimentation Ethics Review Committee. Approval numbers: A1851; Principal investigator: Luchang Shao; Finish date: September 30, 2015 i Statement of contribution of others Financial support for this study was provided by Graduate Research School of James Cook University, JCU Postgraduate Research Scholarship. -

Suborder GOBIOIDEI ELEOTRIDAE Sleepers by E.O
click for previous page 1778 Bony Fishes Suborder GOBIOIDEI ELEOTRIDAE Sleepers by E.O. Murdy, National Science Foundation, Virginia, USA and D.F. Hoese, Australian Museum, Sydney, Australia iagnostic characters: Small to medium-sized (most do not exceed 20 cm, although Gobiomorus from Dthis area may reach 60 cm). Typically, body stout; head short and broad; snout blunt; gill membranes broadly joined to isthmus. Teeth usually small, conical and in several rows in jaws. Six branchiostegal rays. Two separate dorsal fins, first dorsal fin with 6 or 7 weak spines, second dorsal fin with 1 weak spine followed by 6 to 12 soft rays; second dorsal fin and anal fin relatively short-based; origin of anal fin just posterior to vertical with origin of second dorsal fin; terminal ray of second dorsal and anal fins divided to its base (but counted as a single element);anal fin with 1 weak spine followed by 6 to 12 soft rays;caudal fin broad and rounded, compris- ing 15 or 17 segmented rays; pectoral fin broad with 14 to 25 soft rays; pelvic fin long with 1 spine and 5 soft rays.Pelvic fins separate and not connected by a membrane.Scales large and either cycloid or ctenoid.No lateral line on body. Head typically scaled, scales being either cycloid or ctenoid with a series of sensory ca- nals and pores as well as cutaneous papillae. Colour: not brightly coloured, most are light or dark brown or olive with some metallic glints. Habitat, biology, and fisheries: Typically occur in fresh or brackish waters, although some species are truly marine. -

Reef Fisheries and Underwater Surveys Indicate Overfishing of a Brazilian Coastal Island
Research Letters Natureza & Conservação 8(2):151-159, December 2010 Copyright© 2010 ABECO Handling Editor: Sergio R. Floeter Brazilian Journal of Nature Conservation doi: 10.4322/natcon.00802008 Reef Fisheries and Underwater Surveys Indicate Overfishing of a Brazilian Coastal Island Hudson Tercio Pinheiro*, Jean-Christophe Joyeux & Agnaldo Silva Martins Departamento de Oceanografia e Ecologia, Universidade Federal do Espírito Santo, Vitória, ES, Brasil Abstract The preoccupation about fishing effects on marine ecosystems has increased sharply over the last three decades. However, little is known about the impact of multi-gear artisanal and recreational fisheries on the structure of local reef fish communities in Brazil. Fishing activities around a Brazilian coastal island were monitored while reef fish density was censused during underwater surveys (UVC). The links between frequency of capture, intensity at which species are wished and UVC density were explored. Species were classified according to their frequency of capture as regular, occasional and rare, and classified according to the intensity at which they are wished (based on size and price), as highly targeted, average and non-targeted species. Ninety-seven species were caught by fishing, the majority of them being either rarely caught or non-targeted. Nineteen species were highly targeted but rarely caught. The highly targeted species showed extremely low density in the UVC. These results put in question the sustainability of the local fishing activities. The predominance of non-targeted species in the catches and in the reefs environment studied supports the expectation that these species will be more and more captured, thus collaborating to further change the structure of the reef community. -

Identifying Marine Key Biodiversity Areas in the Greater Caribbean Region
Old Dominion University ODU Digital Commons Biological Sciences Theses & Dissertations Biological Sciences Summer 2018 Identifying Marine Key Biodiversity Areas in the Greater Caribbean Region Michael S. Harvey Old Dominion University, [email protected] Follow this and additional works at: https://digitalcommons.odu.edu/biology_etds Part of the Biodiversity Commons, Biology Commons, and the Natural Resources and Conservation Commons Recommended Citation Harvey, Michael S.. "Identifying Marine Key Biodiversity Areas in the Greater Caribbean Region" (2018). Master of Science (MS), Thesis, Biological Sciences, Old Dominion University, DOI: 10.25777/45bp-0v85 https://digitalcommons.odu.edu/biology_etds/32 This Thesis is brought to you for free and open access by the Biological Sciences at ODU Digital Commons. It has been accepted for inclusion in Biological Sciences Theses & Dissertations by an authorized administrator of ODU Digital Commons. For more information, please contact [email protected]. IDENTIFYING MARINE KEY BIODIVERSITY AREAS IN THE GREATER CARIBBEAN REGION by Michael S. Harvey B.A. May 2013, Old Dominion University A Thesis Submitted to the Faculty of Old Dominion University in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE BIOLOGY OLD DOMINION UNIVERSITY August 2018 Approved by: Kent E. Carpenter (Advisor) Beth Polidoro (Member) Sara Maxwell (Member) ABSTRACT IDENTIFYING MARINE KEY BIODIVERSITY AREAS IN THE GREATER CARIBBEAN REGION Michael S. Harvey Old Dominion University, 2018 Advisor: Dr.