Luizjunior Osmarjose M.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fao/Government Cooperative Programme Scientific Basis
FI:GCP/RLA/140/JPN TECHNICAL DOCUMENT No. 4 FAO/GOVERNMENT COOPERATIVE PROGRAMME SCIENTIFIC BASIS FOR ECOSYSTEM-BASED MANAGEMENT IN THE LESSER ANTILLES INCLUDING INTERACTIONS WITH MARINE MAMMALS AND OTHER TOP PREDATORS CRUISE REPORT FOR THE LAPE ECOSYSTEM SURVEY ON RV CELTIC EXPLORER (CE0607) FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Barbados, 2006 FI:GCP/RLA/140/JPN TECHNICAL DOCUMENT No. 4 FAO/GOVERNMENT COOPERATIVE PROGRAMME SCIENTIFIC BASIS FOR ECOSYSTEM-BASED MANAGEMENT IN THE LESSER ANTILLES INCLUDING INTERACTIONS WITH MARINE MAMMALS AND OTHER TOP PREDATORS CRUISE REPORT FOR THE LAPE ECOSYSTEM SURVEY ON RV CELTIC EXPLORER (CE0607) Lesser Antilles Pelagic Ecosystem Project (GCP/RLA/140/JPN) Bridgetown, Barbados FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Barbados, 2006 This technical report is one of a series of reports prepared during the course of the project identified on the title page. The conclusions and recommendations given in the report are those considered appropriate at the time of its preparation. They may be modified in the light of further knowledge gained at subsequent stages of the project. The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries All rights reserved. Reproduction and dissemination of material in this information product for educational or other non-commercial purposes are authorized without any prior written permission from the copyright holders provided the source is fully acknowledged. -

Belonidae Bonaparte 1832 Needlefishes
ISSN 1545-150X California Academy of Sciences A N N O T A T E D C H E C K L I S T S O F F I S H E S Number 16 September 2003 Family Belonidae Bonaparte 1832 needlefishes By Bruce B. Collette National Marine Fisheries Service Systematics Laboratory National Museum of Natural History, Washington, DC 20560–0153, U.S.A. email: [email protected] Needlefishes are a relatively small family of beloniform fishes (Rosen and Parenti 1981 [ref. 5538], Collette et al. 1984 [ref. 11422]) that differ from other members of the order in having both the upper and the lower jaws extended into long beaks filled with sharp teeth (except in the neotenic Belonion), the third pair of upper pharyngeal bones separate, scales on the body relatively small, and no finlets following the dorsal and anal fins. The nostrils lie in a pit anterior to the eyes. There are no spines in the fins. The dorsal fin, with 11–43 rays, and anal fin, with 12–39 rays, are posterior in position; the pelvic fins, with 6 soft rays, are located in an abdominal position; and the pectoral fins are short, with 5–15 rays. The lateral line runs down from the pectoral fin origin and then along the ventral margin of the body. The scales are small, cycloid, and easily detached. Precaudal vertebrae number 33–65, caudal vertebrae 19–41, and total verte- brae 52–97. Some freshwater needlefishes reach only 6 or 7 cm (2.5 or 2.75 in) in total length while some marine species may attain 2 m (6.5 ft). -

Sharkcam Fishes
SharkCam Fishes A Guide to Nekton at Frying Pan Tower By Erin J. Burge, Christopher E. O’Brien, and jon-newbie 1 Table of Contents Identification Images Species Profiles Additional Info Index Trevor Mendelow, designer of SharkCam, on August 31, 2014, the day of the original SharkCam installation. SharkCam Fishes. A Guide to Nekton at Frying Pan Tower. 5th edition by Erin J. Burge, Christopher E. O’Brien, and jon-newbie is licensed under the Creative Commons Attribution-Noncommercial 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc/4.0/. For questions related to this guide or its usage contact Erin Burge. The suggested citation for this guide is: Burge EJ, CE O’Brien and jon-newbie. 2020. SharkCam Fishes. A Guide to Nekton at Frying Pan Tower. 5th edition. Los Angeles: Explore.org Ocean Frontiers. 201 pp. Available online http://explore.org/live-cams/player/shark-cam. Guide version 5.0. 24 February 2020. 2 Table of Contents Identification Images Species Profiles Additional Info Index TABLE OF CONTENTS SILVERY FISHES (23) ........................... 47 African Pompano ......................................... 48 FOREWORD AND INTRODUCTION .............. 6 Crevalle Jack ................................................. 49 IDENTIFICATION IMAGES ...................... 10 Permit .......................................................... 50 Sharks and Rays ........................................ 10 Almaco Jack ................................................. 51 Illustrations of SharkCam -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Atividade De Limpeza E Clientes De Elacatinus Figaro (Pisces: Gobiidae) Nos Recifes De Coral Dos Parrachos De Muriú, Nordeste Do Brasil
Atividade de limpeza e clientes de Elacatinus figaro (Pisces: Gobiidae) nos recifes de coral dos Parrachos de Muriú, Nordeste do Brasil Campos, C.E.C. & Sá-Oliveira, J.C. Biota Neotrop. 2011, 11(1): 47-52. On line version of this paper is available from: http://www.biotaneotropica.org.br/v11n1/en/abstract?article+bn01011012011 A versão on-line completa deste artigo está disponível em: http://www.biotaneotropica.org.br/v11n1/pt/abstract?article+bn01011012011 Received/ Recebido em 30/06/2010 - Revised/ Versão reformulada recebida em 14/12/2010 - Accepted/ Publicado em 11/01/2011 ISSN 1676-0603 (on-line) Biota Neotropica is an electronic, peer-reviewed journal edited by the Program BIOTA/FAPESP: The Virtual Institute of Biodiversity. This journal’s aim is to disseminate the results of original research work, associated or not to the program, concerned with characterization, conservation and sustainable use of biodiversity within the Neotropical region. Biota Neotropica é uma revista do Programa BIOTA/FAPESP - O Instituto Virtual da Biodiversidade, que publica resultados de pesquisa original, vinculada ou não ao programa, que abordem a temática caracterização, conservação e uso sustentável da biodiversidade na região Neotropical. Biota Neotropica is an eletronic journal which is available free at the following site http://www.biotaneotropica.org.br A Biota Neotropica é uma revista eletrônica e está integral e gratuitamente disponível no endereço http://www.biotaneotropica.org.br Biota Neotrop., vol. 11, no. 1 Atividade de limpeza e clientes de Elacatinus figaro (Pisces: Gobiidae) nos recifes de coral dos Parrachos de Muriú, Nordeste do Brasil Carlos Eduardo Costa Campos1,2 & Júlio César Sá-Oliveira1 1 Laboratório de Zoologia, Departamento de Ciências Biológicas, Universidade Federal do Amapá – UNIFAP, Rod. -

Herbivory by the Dusky Damselfish Stegastes Fuscus (Cuvier, 1830)
Journal of Experimental Marine Biology and Ecology, L 229 (1998) 241±264 Herbivory by the Dusky Damsel®sh Stegastes fuscus (Cuvier, 1830) in a tropical rocky shore: effects on the benthic community Carlos Eduardo L. Ferreiraa,b,* , Jose Eduardo A. GoncËalves b , Ricardo Coutinhoba , Alberto C. Peret aDep. de Hidrobiologia, Universidade Federal de SaoÄÄ Carlos, Sao Carlos, S.P., Cep:13560 000, Brazil bDept. de Biologia, Instituto de Estudos do Mar Almirante Paulo Moreira, Arraial do Cabo, R.J., Cep: 28930 000, Brazil Received 12 September 1995; received in revised form 31 March 1997; accepted 13 March 1998 Abstract Experiments were carried out on rocky shores at Arraial do Cabo (Southeast Brazil) to evaluate how the dusky damsel®sh, Stegastes fuscus (Cuvier, 1830) affects the benthic community structure. Cage exclusion showed that S. fuscus strongly in¯uences the algal community in its territories, keeping it in an early succession stage and preventing dominance by Jania spp. Diversity and biomass of the epilithic algal community (EAC) were higher inside territories than | 2 outside. These dense mats hold a diverse and abundant cryptofauna (5 72 ind/100 cm ) that was signi®cantly higher inside territories. Algae comprise 70% of the S. fuscus diet, with the remaining 30% composed of animal material. The ®sh feeds selectively mainly on red ®lamentous algae, such as Polysiphonia spp., Ceramium spp. and Centroceras clavulatum, albeit it also ingests a great amount of calcareous algae (25% of total algae). Total assimilation and nitrogen assimilation were low in S. fuscus. Gut contents turnover varied from 3.7 in summer to 4.1 in winter. -

PROCEEDINGS of the BIOLOGICAL SOCIETY of WASHINGTON 115(L):32-50
PROCEEDINGS OF THE BIOLOGICAL SOCIETY OF WASHINGTON 115(l):32-50. 2002. Revision of Atlantic sharpnose pufferfishes (Tetraodontiformes: Tetraodontidae: Canthigaster), with description of three new species Rodrigo L. Moura and Ricardo M. C. Castro (RLM) Segao de Peixes, Museu de Zoologia, Universidade de Sao Paulo CP 42694, Sao Paulo, SP 04299-970, Brazil, e-mail: [email protected]; (RMCC) Laboratorio de Ictiologia, Departamento de Biologia, FFCLRP, Universidade de Sao Paulo, Av. Bandeirantes 3900, Ribeirao Preto, SP 14040-901, Brazil Abstract. —Six species of sharpnose puffers are herein recognized from the Atlantic Ocean, three of which are described as new: Canthigaster figueiredoi, n. sp. from the east coast of South America, Canthigaster jamestyleri, n. sp. from deep reefs off the southeast coast of the United States and the Gulf of Mexico, and Canthigaster supramacula, n. sp. from the west coast of Africa. Canthigaster capistratus (Lowe 1839), described from the Madeira Islands and previously considered to be a junior synonym of C. rostrata (Bloch, 1786), is revalidated and redescribed; it's known distribution extends from the Macaro- nesian Region to the Mediterranean. Canthigaster rostrata (Bloch, 1786), re- stricted to shallow-water northwestern Atlantic reefs, and C sanctaehelenae (Giinther, 1870), endemic to the mid-Atlantic islands of Ascension and St. Helena, also are diagnosed and redescribed. An identification key based on pigment pattern features is provided for all six Atlantic species of Canthigaster. Resumo.—Seis especies do genero Canthigaster (Tetraodontidae: Canthigas- terinae) sao reconhecidas no Oceano Atlantico, tres das quais sao descritas como novas: Canthigaster figueiredoi sp. n., da costa oriental da America do Sul, Canthigaster jamestyleri sp.n., de recifes profundos da costa Sudeste dos Estados Unidos e Golfo do Mexico e, por fim, Canthigaster supramacula sp. -

Ecomorfologia E Padrões De Abundância De Labridae Em Ilhas Oceânicas E Áreas Da Costa Brasileira
UNIVERSIDADE FEDERAL DO ESPÍRITO SANTO CENTRO DE CIÊNCIAS HUMANAS E NATURAIS PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS BIOLÓGICAS Ecomorfologia e padrões de abundância de Labridae em ilhas oceânicas e áreas da costa brasileira Gabriel Costa Cardozo Ferreira Vitória, ES Fevereiro, 2015 UNIVERSIDADE FEDERAL DO ESPÍRITO SANTO CENTRO DE CIÊNCIAS HUMANAS E NATURAIS PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS BIOLÓGICAS Ecomorfologia e padrões de abundância de Labridae em ilhas oceânicas e áreas da costa brasileira Gabriel Costa Cardozo Ferreira Orientador: Jean-Christophe Joyeux Co-orientador: Ronaldo Bastos Francini-Filho Dissertação submetida ao Programa de Pós-Graduação em Ciências Biológicas (Biologia Animal) da Universidade Federal do Espírito Santo como requisito parcial para a obtenção do grau de Mestre em Biologia Animal Vitória, ES Fevereiro, 2015 SUMÁRIO Introdução Geral .................................................................................................................. 11 Referências ...................................................................................................................... 14 Capítulo 1 ............................................................................................................................ 18 Variações ontogenéticas em grupos funcionais de Labridae do Atlântico sudoeste ............. 18 Resumo ............................................................................................................................ 18 Abstract ........................................................................................................................... -

Universidade Federal Do Estado Do Rio De Janeiro Instituto De Biociências
1 UNIVERSIDADE FEDERAL DO ESTADO DO RIO DE JANEIRO INSTITUTO DE BIOCIÊNCIAS COMPORTAMENTO TERRITORIALISTA DO Stegastes fuscus (Cuvier, 1830) E A ASSEMBLEIA DE PEIXES RECIFAIS EM COSTÃO ROCHOSO TROPICAL: ANÁLISE DE SENSIBILIDADE Victor Bastos Teixeira Lupinacci Rio de Janeiro 2019 2 Victor Bastos Teixeira Lupinacci COMPORTAMENTO TERRITORIALISTA DO Stegastes fuscus (Cuvier, 1830) E A ASSEMBLEIA DE PEIXES RECIFAIS EM COSTÃO ROCHOSO TROPICAL: ANÁLISE DE SENSIBILIDADE Monografia do Trabalho de Conclusão de Curso apresentada ao Instituto de Biociências da Universidade Federal do Estado do Rio de Janeiro, como parte dos requisitos à obtenção do título de Bacharel em Ciências Ambientais. Orientador: Prof. Dr. Rafael da Rocha Fortes Rio de Janeiro 2019 3 LUPINACCI, Victor COMPORTAMENTO TERRITORIALISTA DO Stegastes fuscus (Cuvier, 1830) E A ASSEMBLEIA DE PEIXES RECIFAIS EM COSTÃO ROCHOSO TROPICAL: ANÁLISE DE SENSIBILIDADE. - 2019. Monografia do Trabalho de Conclusão de Curso Orientador: Prof. Dr. Rafael da Rocha Fortes 1. Territorialidade 2. Ambientes recifais 3. Comportamento agonístico I. Universidade Federal do Estado do Rio de Janeiro II. Comportamento Territorialista do Stegastes fuscus (Cuvier, 1830) e Riqueza de Peixes Recifais em Costão Rochoso Tropical: Análise de Sensibilidade 4 Victor Bastos Teixeira Lupinacci COMPORTAMENTO TERRITORIALISTA DO Stegastes fuscus (Cuvier, 1830) E A ASSEMBLEIA DE PEIXES RECIFAIS EM COSTÃO ROCHOSO TROPICAL: ANÁLISE DE SENSIBILIDADE - 2019. Monografia do Trabalho de Conclusão de Curso apresentada ao Instituto de Biociências da Universidade Federal do Estado do Rio de Janeiro, como parte dos requisitos à obtenção do título de Bacharel em Ciências Ambientais. Aprovada em ___ de _____________ de ______. ____________________________________________________ Prof. Dr. Rafael da Rocha Fortes (Orientador) Universidade Federal do Estado do Rio de Janeiro - UNIRIO ____________________________________________________ Dr. -
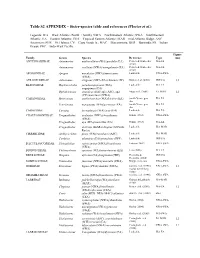
Table S2 APPENDIX – Sister-Species Table and References (Floeter Et Al.)
Table S2 APPENDIX – Sister-species table and references (Floeter et al.) Legends: WA = West Atlantic (North + South); NWA = Northwestern Atlantic; SWA = Southwestern Atlantic; EA = Eastern Atlantic; TEA = Tropical Eastern Atlantic; MAR = mid-Atlantic Ridge; ASC = Ascension; SHE = St. Helena; CV = Cape Verde Is.; MAC = Macaronesia; BER = Bermuda; IO = Indian Ocean; IWP = Indo-West Pacific. Figure Family Genus Species Reference Type (ms) ANTENNARIIDAE Antennarius multiocellatus (WA)/pardalis (EA) Pietsch & Grobecker WA-EA (1987) Antennarius ocellatus (NWA)/senegalensis (EA) Pietsch & Grobecker WA-EA (1987) APOGONIDAE Apogon maculatus (NWA)/americanus Look alike NWA-SWA (SWA) AULOSTOMIDAE Aulostomus strigosus (SWA-EA)/chinensis (IP) Bowen et al. (2001) IWP-EA 12 BLENNIIDAE Hypleurochilus pseudoaequipinnis (WA)/ Look alike WA-EA aequipinnis (EA) Ophioblennius atlanticus (EA)/ sp1 (ASC), sp2 Muss et al. (2001) EA-MAR 12 (CV)/macclurei (NWA) CARANGIDAE Hemicaranx amblyrhynchus (WA)/bicolor (EA) Smith-Vaniz, pers WA-EA obs. Trachinotus marginatus (WA)/goreensis (EA) Smith-Vaniz, pers WA-EA obs. CARAPIDAE Carapus bermudensis (WA)/acus (EA) Look alike WA-EA CHAETODONTIDAE Prognathodes aculeatus (NWA)/brasiliensis Hubbs (1963) NWA-SWA (SWA) Prognathodes aya (WA)/marcellae (EA) Hubbs (1963) WA-EA Prognathodes dichrous (MAR)/obliquus (St Paul's Look alike WA-MAR Rocks) CIRRHITIDAE Amblycirrhitus pinos (WA)/earnshawi (ASC) Look alike WA-MAR Cirrhitus atlanticus (EA)/pinnulatus (IWP) Look alike IWP-EA DACTYLOSCOPIDAE Platygillellus rubrocinctus (NWA)/brasiliensis Feitoza (2002) NWA-SWA (SWA) DIODONTIDAE Chilomycterus spinosus (WA)/mauretanicus (EA) Leis (2006) WA-EA DREPANIDAE Drepane africana (EA)/longimana (IWP) Heemstra & IWP-EA Heemstra (2004) GOBIESOCIDAE Tomicodon fasciatus (NWA)/australis (SWA) Briggs, pers com NWA-SWA GOBIIDAE Elacatinus figaro (SWA)/randalli (NWA) Sazima et al. -

Hotspots, Extinction Risk and Conservation Priorities of Greater Caribbean and Gulf of Mexico Marine Bony Shorefishes
Old Dominion University ODU Digital Commons Biological Sciences Theses & Dissertations Biological Sciences Summer 2016 Hotspots, Extinction Risk and Conservation Priorities of Greater Caribbean and Gulf of Mexico Marine Bony Shorefishes Christi Linardich Old Dominion University, [email protected] Follow this and additional works at: https://digitalcommons.odu.edu/biology_etds Part of the Biodiversity Commons, Biology Commons, Environmental Health and Protection Commons, and the Marine Biology Commons Recommended Citation Linardich, Christi. "Hotspots, Extinction Risk and Conservation Priorities of Greater Caribbean and Gulf of Mexico Marine Bony Shorefishes" (2016). Master of Science (MS), Thesis, Biological Sciences, Old Dominion University, DOI: 10.25777/hydh-jp82 https://digitalcommons.odu.edu/biology_etds/13 This Thesis is brought to you for free and open access by the Biological Sciences at ODU Digital Commons. It has been accepted for inclusion in Biological Sciences Theses & Dissertations by an authorized administrator of ODU Digital Commons. For more information, please contact [email protected]. HOTSPOTS, EXTINCTION RISK AND CONSERVATION PRIORITIES OF GREATER CARIBBEAN AND GULF OF MEXICO MARINE BONY SHOREFISHES by Christi Linardich B.A. December 2006, Florida Gulf Coast University A Thesis Submitted to the Faculty of Old Dominion University in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE BIOLOGY OLD DOMINION UNIVERSITY August 2016 Approved by: Kent E. Carpenter (Advisor) Beth Polidoro (Member) Holly Gaff (Member) ABSTRACT HOTSPOTS, EXTINCTION RISK AND CONSERVATION PRIORITIES OF GREATER CARIBBEAN AND GULF OF MEXICO MARINE BONY SHOREFISHES Christi Linardich Old Dominion University, 2016 Advisor: Dr. Kent E. Carpenter Understanding the status of species is important for allocation of resources to redress biodiversity loss. -

FISHES (C) Val Kells–November, 2019
VAL KELLS Marine Science Illustration 4257 Ballards Mill Road - Free Union - VA - 22940 www.valkellsillustration.com [email protected] STOCK ILLUSTRATION LIST FRESHWATER and SALTWATER FISHES (c) Val Kells–November, 2019 Eastern Atlantic and Gulf of Mexico: brackish and saltwater fishes Subject to change. New illustrations added weekly. Atlantic hagfish, Myxine glutinosa Sea lamprey, Petromyzon marinus Deepwater chimaera, Hydrolagus affinis Atlantic spearnose chimaera, Rhinochimaera atlantica Nurse shark, Ginglymostoma cirratum Whale shark, Rhincodon typus Sand tiger, Carcharias taurus Ragged-tooth shark, Odontaspis ferox Crocodile Shark, Pseudocarcharias kamoharai Thresher shark, Alopias vulpinus Bigeye thresher, Alopias superciliosus Basking shark, Cetorhinus maximus White shark, Carcharodon carcharias Shortfin mako, Isurus oxyrinchus Longfin mako, Isurus paucus Porbeagle, Lamna nasus Freckled Shark, Scyliorhinus haeckelii Marbled catshark, Galeus arae Chain dogfish, Scyliorhinus retifer Smooth dogfish, Mustelus canis Smalleye Smoothhound, Mustelus higmani Dwarf Smoothhound, Mustelus minicanis Florida smoothhound, Mustelus norrisi Gulf Smoothhound, Mustelus sinusmexicanus Blacknose shark, Carcharhinus acronotus Bignose shark, Carcharhinus altimus Narrowtooth Shark, Carcharhinus brachyurus Spinner shark, Carcharhinus brevipinna Silky shark, Carcharhinus faiformis Finetooth shark, Carcharhinus isodon Galapagos Shark, Carcharhinus galapagensis Bull shark, Carcharinus leucus Blacktip shark, Carcharhinus limbatus Oceanic whitetip shark,