2016 Tese Lmdegurjao.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CAT Vertebradosgt CDC CECON USAC 2019
Catálogo de Autoridades Taxonómicas de vertebrados de Guatemala CDC-CECON-USAC 2019 Centro de Datos para la Conservación (CDC) Centro de Estudios Conservacionistas (Cecon) Facultad de Ciencias Químicas y Farmacia Universidad de San Carlos de Guatemala Este documento fue elaborado por el Centro de Datos para la Conservación (CDC) del Centro de Estudios Conservacionistas (Cecon) de la Facultad de Ciencias Químicas y Farmacia de la Universidad de San Carlos de Guatemala. Guatemala, 2019 Textos y edición: Manolo J. García. Zoólogo CDC Primera edición, 2019 Centro de Estudios Conservacionistas (Cecon) de la Facultad de Ciencias Químicas y Farmacia de la Universidad de San Carlos de Guatemala ISBN: 978-9929-570-19-1 Cita sugerida: Centro de Estudios Conservacionistas [Cecon]. (2019). Catálogo de autoridades taxonómicas de vertebrados de Guatemala (Documento técnico). Guatemala: Centro de Datos para la Conservación [CDC], Centro de Estudios Conservacionistas [Cecon], Facultad de Ciencias Químicas y Farmacia, Universidad de San Carlos de Guatemala [Usac]. Índice 1. Presentación ............................................................................................ 4 2. Directrices generales para uso del CAT .............................................. 5 2.1 El grupo objetivo ..................................................................... 5 2.2 Categorías taxonómicas ......................................................... 5 2.3 Nombre de autoridades .......................................................... 5 2.4 Estatus taxonómico -

Andrea RAZ-GUZMÁN1*, Leticia HUIDOBRO2, and Virginia PADILLA3
ACTA ICHTHYOLOGICA ET PISCATORIA (2018) 48 (4): 341–362 DOI: 10.3750/AIEP/02451 AN UPDATED CHECKLIST AND CHARACTERISATION OF THE ICHTHYOFAUNA (ELASMOBRANCHII AND ACTINOPTERYGII) OF THE LAGUNA DE TAMIAHUA, VERACRUZ, MEXICO Andrea RAZ-GUZMÁN1*, Leticia HUIDOBRO2, and Virginia PADILLA3 1 Posgrado en Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México, Ciudad de México 2 Instituto Nacional de Pesca y Acuacultura, SAGARPA, Ciudad de México 3 Facultad de Ciencias, Universidad Nacional Autónoma de México, Ciudad de México Raz-Guzmán A., Huidobro L., Padilla V. 2018. An updated checklist and characterisation of the ichthyofauna (Elasmobranchii and Actinopterygii) of the Laguna de Tamiahua, Veracruz, Mexico. Acta Ichthyol. Piscat. 48 (4): 341–362. Background. Laguna de Tamiahua is ecologically and economically important as a nursery area that favours the recruitment of species that sustain traditional fisheries. It has been studied previously, though not throughout its whole area, and considering the variety of habitats that sustain these fisheries, as well as an increase in population growth that impacts the system. The objectives of this study were to present an updated list of fish species, data on special status, new records, commercial importance, dominance, density, ecotic position, and the spatial and temporal distribution of species in the lagoon, together with a comparison of Tamiahua with 14 other Gulf of Mexico lagoons. Materials and methods. Fish were collected in August and December 1996 with a Renfro beam net and an otter trawl from different habitats throughout the lagoon. The species were identified, classified in relation to special status, new records, commercial importance, density, dominance, ecotic position, and spatial distribution patterns. -

Community Structure of Reef Fishes on a Remote Oceanic Island
CSIRO PUBLISHING Marine and Freshwater Research http://dx.doi.org/10.1071/MF14150 Community structure of reef fishes on a remote oceanic island (St Peter and St Paul’s Archipelago, equatorial Atlantic): the relative influence of abiotic and biotic variables Osmar J. LuizA,G, Thiago C. MendesB, Diego R. BarnecheA, Carlos G. W. FerreiraC, Ramon NoguchiD, Roberto C. Villac¸aB, Carlos A. RangelE, Joa˜o L. GaspariniF and Carlos E. L. FerreiraB ADepartment of Biological Sciences, Macquarie University, Sydney, NSW 2109, Australia. BDepartamento de Biologia Marinha, Universidade Federal Fluminense, Nitero´i, RJ, 24001-970, Brazil. CDepartamento de Oceanografia, Instituto de Estudos do Mar Almirante Paulo Moreira, Arraial do Cabo, RJ, 28930-000, Brazil. DPrograma de Po´s Graduac¸a˜o em Ecologia, Universidade Federal de Rio de Janeiro, Rio de Janeiro, RJ, 68020, Brazil. EProjeto Ilhas do Rio, Instituto Mar Adentro, Rio de Janeiro, RJ, 22031-071, Brazil. FDepartamento de Oceanografia e Ecologia, Universidade Federal do Espı´rito Santo, Vito´ria, ES, Brazil. GCorresponding author. Email: [email protected] Abstract. This study investigates the reef fish community structure of the world’s smallest remote tropical island, the St Peter and St Paul’s Archipelago, in the equatorial Atlantic. The interplay between isolation, high endemism and low species richness makes the St Peter and St Paul’s Archipelago ecologically simpler than larger and highly connected shelf reef systems, making it an important natural laboratory for ecology and biogeography, particularly with respect to the effects of abiotic and biotic factors, and the functional organisation of such a depauperate community. Boosted regression trees were used to associate density, biomass and diversity of reef fishes with six abiotic and biotic variables, considering the community both as a whole and segregated into seven trophic groups. -

Atividade De Limpeza E Clientes De Elacatinus Figaro (Pisces: Gobiidae) Nos Recifes De Coral Dos Parrachos De Muriú, Nordeste Do Brasil
Atividade de limpeza e clientes de Elacatinus figaro (Pisces: Gobiidae) nos recifes de coral dos Parrachos de Muriú, Nordeste do Brasil Campos, C.E.C. & Sá-Oliveira, J.C. Biota Neotrop. 2011, 11(1): 47-52. On line version of this paper is available from: http://www.biotaneotropica.org.br/v11n1/en/abstract?article+bn01011012011 A versão on-line completa deste artigo está disponível em: http://www.biotaneotropica.org.br/v11n1/pt/abstract?article+bn01011012011 Received/ Recebido em 30/06/2010 - Revised/ Versão reformulada recebida em 14/12/2010 - Accepted/ Publicado em 11/01/2011 ISSN 1676-0603 (on-line) Biota Neotropica is an electronic, peer-reviewed journal edited by the Program BIOTA/FAPESP: The Virtual Institute of Biodiversity. This journal’s aim is to disseminate the results of original research work, associated or not to the program, concerned with characterization, conservation and sustainable use of biodiversity within the Neotropical region. Biota Neotropica é uma revista do Programa BIOTA/FAPESP - O Instituto Virtual da Biodiversidade, que publica resultados de pesquisa original, vinculada ou não ao programa, que abordem a temática caracterização, conservação e uso sustentável da biodiversidade na região Neotropical. Biota Neotropica is an eletronic journal which is available free at the following site http://www.biotaneotropica.org.br A Biota Neotropica é uma revista eletrônica e está integral e gratuitamente disponível no endereço http://www.biotaneotropica.org.br Biota Neotrop., vol. 11, no. 1 Atividade de limpeza e clientes de Elacatinus figaro (Pisces: Gobiidae) nos recifes de coral dos Parrachos de Muriú, Nordeste do Brasil Carlos Eduardo Costa Campos1,2 & Júlio César Sá-Oliveira1 1 Laboratório de Zoologia, Departamento de Ciências Biológicas, Universidade Federal do Amapá – UNIFAP, Rod. -

Herbivory by the Dusky Damselfish Stegastes Fuscus (Cuvier, 1830)
Journal of Experimental Marine Biology and Ecology, L 229 (1998) 241±264 Herbivory by the Dusky Damsel®sh Stegastes fuscus (Cuvier, 1830) in a tropical rocky shore: effects on the benthic community Carlos Eduardo L. Ferreiraa,b,* , Jose Eduardo A. GoncËalves b , Ricardo Coutinhoba , Alberto C. Peret aDep. de Hidrobiologia, Universidade Federal de SaoÄÄ Carlos, Sao Carlos, S.P., Cep:13560 000, Brazil bDept. de Biologia, Instituto de Estudos do Mar Almirante Paulo Moreira, Arraial do Cabo, R.J., Cep: 28930 000, Brazil Received 12 September 1995; received in revised form 31 March 1997; accepted 13 March 1998 Abstract Experiments were carried out on rocky shores at Arraial do Cabo (Southeast Brazil) to evaluate how the dusky damsel®sh, Stegastes fuscus (Cuvier, 1830) affects the benthic community structure. Cage exclusion showed that S. fuscus strongly in¯uences the algal community in its territories, keeping it in an early succession stage and preventing dominance by Jania spp. Diversity and biomass of the epilithic algal community (EAC) were higher inside territories than | 2 outside. These dense mats hold a diverse and abundant cryptofauna (5 72 ind/100 cm ) that was signi®cantly higher inside territories. Algae comprise 70% of the S. fuscus diet, with the remaining 30% composed of animal material. The ®sh feeds selectively mainly on red ®lamentous algae, such as Polysiphonia spp., Ceramium spp. and Centroceras clavulatum, albeit it also ingests a great amount of calcareous algae (25% of total algae). Total assimilation and nitrogen assimilation were low in S. fuscus. Gut contents turnover varied from 3.7 in summer to 4.1 in winter. -

PROCEEDINGS of the BIOLOGICAL SOCIETY of WASHINGTON 115(L):32-50
PROCEEDINGS OF THE BIOLOGICAL SOCIETY OF WASHINGTON 115(l):32-50. 2002. Revision of Atlantic sharpnose pufferfishes (Tetraodontiformes: Tetraodontidae: Canthigaster), with description of three new species Rodrigo L. Moura and Ricardo M. C. Castro (RLM) Segao de Peixes, Museu de Zoologia, Universidade de Sao Paulo CP 42694, Sao Paulo, SP 04299-970, Brazil, e-mail: [email protected]; (RMCC) Laboratorio de Ictiologia, Departamento de Biologia, FFCLRP, Universidade de Sao Paulo, Av. Bandeirantes 3900, Ribeirao Preto, SP 14040-901, Brazil Abstract. —Six species of sharpnose puffers are herein recognized from the Atlantic Ocean, three of which are described as new: Canthigaster figueiredoi, n. sp. from the east coast of South America, Canthigaster jamestyleri, n. sp. from deep reefs off the southeast coast of the United States and the Gulf of Mexico, and Canthigaster supramacula, n. sp. from the west coast of Africa. Canthigaster capistratus (Lowe 1839), described from the Madeira Islands and previously considered to be a junior synonym of C. rostrata (Bloch, 1786), is revalidated and redescribed; it's known distribution extends from the Macaro- nesian Region to the Mediterranean. Canthigaster rostrata (Bloch, 1786), re- stricted to shallow-water northwestern Atlantic reefs, and C sanctaehelenae (Giinther, 1870), endemic to the mid-Atlantic islands of Ascension and St. Helena, also are diagnosed and redescribed. An identification key based on pigment pattern features is provided for all six Atlantic species of Canthigaster. Resumo.—Seis especies do genero Canthigaster (Tetraodontidae: Canthigas- terinae) sao reconhecidas no Oceano Atlantico, tres das quais sao descritas como novas: Canthigaster figueiredoi sp. n., da costa oriental da America do Sul, Canthigaster jamestyleri sp.n., de recifes profundos da costa Sudeste dos Estados Unidos e Golfo do Mexico e, por fim, Canthigaster supramacula sp. -

Gadiformes: Moridae), with Description of a New Species from Saint Peter and Saint Paul Archipelago, Equatorial Atlantic
Zootaxa 4671 (1): 067–080 ISSN 1175-5326 (print edition) https://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2019 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4671.1.5 http://zoobank.org/urn:lsid:zoobank.org:pub:2DAC5AD3-6DAA-4D57-9FF0-C024174BAC0C Review of the Brazilian species of Physiculus (Gadiformes: Moridae), with description of a new species from Saint Peter and Saint Paul Archipelago, equatorial Atlantic ALESSANDRA M. A. PIRES1, ALFREDO CARVALHO-FILHO2,4, RÔMULO C. P. FERREIRA1, DANIELLE VIANA1, DIOGO NUNES3 & FABIO H. V. HAZIN1. ¹Universidade Federal Rural de Pernambuco, Rua Dom Manuel de Medeiros, s/n, Dois Irmãos, 52171-900, Recife, PE, Brasil E-mail: [email protected] ²Fish Bizz Ltda. Rua Moncorvo Filho 51, 05424-070, São Paulo, SP, Brasil. E-mail: [email protected] 3Unidade Acadêmica de Serra Talhada, Universidade Federal Rural de Pernambuco Fazenda Saco, Serra Talhada-PE, Brasil. 4Corresponding author Abstract Three valid species of the genus Physiculus are known from the Brazilian marine waters. A fourth, new species, Physiculus cirm n. sp.., is described based on seventeen specimens collected in the surroundings of Saint Peter and Saint Paul Archipelago, equatorial Atlantic. A review of the Brazilian species of Physiculus is provided, as well as a key to the species of the genus reported from the Atlantic Ocean. The new species is distinguished from all its congeners, except P. cynodon and P. karrerae, by the large number of longitudinal series of scales (156–189 vs. 70–150). P. cynodon from the Northern Pacific has about 200 longitudinal series of scales, and it differs from the new species by the number of rays of the first dorsal fin (6–8 vs. -

PEIXES RECIFAIS DO NORDESTE BRASILEIRO.Pdf
UNIVERSIDADE FEDERAL DA PARAÍBA CENTRO DE CIÊNCIAS EXATAS E DA NATUREZA CURSO DE BACHARELADO EM CIÊNCIAS BIOLÓGICAS DIVERSIDADE DOS PEIXES RECIFAIS DO NORDESTE BRASILEIRO Jessé Miranda de Figueiredo Filho Ricardo de Souza Rosa Orientador João Pessoa – 2016 UNIVERSIDADE FEDERAL DA PARAÍBA CENTRO DE CIÊNCIAS EXATAS E DA NATUREZA CURSO DE BACHARELADO EM CIÊNCIAS BIOLÓGICAS DIVERSIDADE DOS PEIXES RECIFAIS DO NORDESTE BRASILEIRO Jessé Miranda de Figueiredo Filho Ricardo de Souza Rosa Orientador Monografia apresentada ao Curso de Ciências Biológicas como requisito parcial à obtenção do grau de Bacharel em Ciências Biológicas. João Pessoa – 2016 Catalogação na publicação Universidade Federal da Paraíba Biblioteca Setorial do CCEN Maria Teresa Macau - CRB 15/176 F475d Figueiredo Filho, Jessé Miranda de. Diversidade dos peixes recifais do Nordeste Brasileiro / Jessé Miranda de Figueiredo Filho. – João Pessoa, 2016. 40p. : il.- Monografia ( Bacharelado em Ciências Biológicas ) – Universidade Federal da Paraíba. Orientador: Profº Drº Ricardo de Souza Rosa. 1. Peixes. 2. Recifes. 3. Lista sistemática. I. Título. CDU : 597.2/.5(043.2) “By the deep sea, and music in its roar I love not man the less, but nature more.” (George Gordon Byron) AGRADECIMENTOS À minha família – em especial aos meus pais, Jessé Figueiredo e Tânia Ribeiro, por todo o apoio, confiança, carinho e conselhos que depositaram em mim durante toda a minha vida, pela ajuda nos momentos difíceis e comemorações nas alegrias da vida, sem vocês eu não teria conseguido. À minha irmã, Tayná Ribeiro, pelo apoio, sinceridade, discussões, e por sempre acreditar em mim. Ao meu orientador, Dr. Ricardo Rosa, pela orientação, paciência e confiança; por ter me apresentado ao mundo fantástico da ictiologia, e acreditado no meu potencial. -

Universidade Federal Do Estado Do Rio De Janeiro Instituto De Biociências
1 UNIVERSIDADE FEDERAL DO ESTADO DO RIO DE JANEIRO INSTITUTO DE BIOCIÊNCIAS COMPORTAMENTO TERRITORIALISTA DO Stegastes fuscus (Cuvier, 1830) E A ASSEMBLEIA DE PEIXES RECIFAIS EM COSTÃO ROCHOSO TROPICAL: ANÁLISE DE SENSIBILIDADE Victor Bastos Teixeira Lupinacci Rio de Janeiro 2019 2 Victor Bastos Teixeira Lupinacci COMPORTAMENTO TERRITORIALISTA DO Stegastes fuscus (Cuvier, 1830) E A ASSEMBLEIA DE PEIXES RECIFAIS EM COSTÃO ROCHOSO TROPICAL: ANÁLISE DE SENSIBILIDADE Monografia do Trabalho de Conclusão de Curso apresentada ao Instituto de Biociências da Universidade Federal do Estado do Rio de Janeiro, como parte dos requisitos à obtenção do título de Bacharel em Ciências Ambientais. Orientador: Prof. Dr. Rafael da Rocha Fortes Rio de Janeiro 2019 3 LUPINACCI, Victor COMPORTAMENTO TERRITORIALISTA DO Stegastes fuscus (Cuvier, 1830) E A ASSEMBLEIA DE PEIXES RECIFAIS EM COSTÃO ROCHOSO TROPICAL: ANÁLISE DE SENSIBILIDADE. - 2019. Monografia do Trabalho de Conclusão de Curso Orientador: Prof. Dr. Rafael da Rocha Fortes 1. Territorialidade 2. Ambientes recifais 3. Comportamento agonístico I. Universidade Federal do Estado do Rio de Janeiro II. Comportamento Territorialista do Stegastes fuscus (Cuvier, 1830) e Riqueza de Peixes Recifais em Costão Rochoso Tropical: Análise de Sensibilidade 4 Victor Bastos Teixeira Lupinacci COMPORTAMENTO TERRITORIALISTA DO Stegastes fuscus (Cuvier, 1830) E A ASSEMBLEIA DE PEIXES RECIFAIS EM COSTÃO ROCHOSO TROPICAL: ANÁLISE DE SENSIBILIDADE - 2019. Monografia do Trabalho de Conclusão de Curso apresentada ao Instituto de Biociências da Universidade Federal do Estado do Rio de Janeiro, como parte dos requisitos à obtenção do título de Bacharel em Ciências Ambientais. Aprovada em ___ de _____________ de ______. ____________________________________________________ Prof. Dr. Rafael da Rocha Fortes (Orientador) Universidade Federal do Estado do Rio de Janeiro - UNIRIO ____________________________________________________ Dr. -
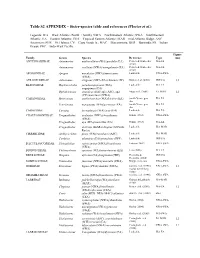
Table S2 APPENDIX – Sister-Species Table and References (Floeter Et Al.)
Table S2 APPENDIX – Sister-species table and references (Floeter et al.) Legends: WA = West Atlantic (North + South); NWA = Northwestern Atlantic; SWA = Southwestern Atlantic; EA = Eastern Atlantic; TEA = Tropical Eastern Atlantic; MAR = mid-Atlantic Ridge; ASC = Ascension; SHE = St. Helena; CV = Cape Verde Is.; MAC = Macaronesia; BER = Bermuda; IO = Indian Ocean; IWP = Indo-West Pacific. Figure Family Genus Species Reference Type (ms) ANTENNARIIDAE Antennarius multiocellatus (WA)/pardalis (EA) Pietsch & Grobecker WA-EA (1987) Antennarius ocellatus (NWA)/senegalensis (EA) Pietsch & Grobecker WA-EA (1987) APOGONIDAE Apogon maculatus (NWA)/americanus Look alike NWA-SWA (SWA) AULOSTOMIDAE Aulostomus strigosus (SWA-EA)/chinensis (IP) Bowen et al. (2001) IWP-EA 12 BLENNIIDAE Hypleurochilus pseudoaequipinnis (WA)/ Look alike WA-EA aequipinnis (EA) Ophioblennius atlanticus (EA)/ sp1 (ASC), sp2 Muss et al. (2001) EA-MAR 12 (CV)/macclurei (NWA) CARANGIDAE Hemicaranx amblyrhynchus (WA)/bicolor (EA) Smith-Vaniz, pers WA-EA obs. Trachinotus marginatus (WA)/goreensis (EA) Smith-Vaniz, pers WA-EA obs. CARAPIDAE Carapus bermudensis (WA)/acus (EA) Look alike WA-EA CHAETODONTIDAE Prognathodes aculeatus (NWA)/brasiliensis Hubbs (1963) NWA-SWA (SWA) Prognathodes aya (WA)/marcellae (EA) Hubbs (1963) WA-EA Prognathodes dichrous (MAR)/obliquus (St Paul's Look alike WA-MAR Rocks) CIRRHITIDAE Amblycirrhitus pinos (WA)/earnshawi (ASC) Look alike WA-MAR Cirrhitus atlanticus (EA)/pinnulatus (IWP) Look alike IWP-EA DACTYLOSCOPIDAE Platygillellus rubrocinctus (NWA)/brasiliensis Feitoza (2002) NWA-SWA (SWA) DIODONTIDAE Chilomycterus spinosus (WA)/mauretanicus (EA) Leis (2006) WA-EA DREPANIDAE Drepane africana (EA)/longimana (IWP) Heemstra & IWP-EA Heemstra (2004) GOBIESOCIDAE Tomicodon fasciatus (NWA)/australis (SWA) Briggs, pers com NWA-SWA GOBIIDAE Elacatinus figaro (SWA)/randalli (NWA) Sazima et al. -

Hotspots, Extinction Risk and Conservation Priorities of Greater Caribbean and Gulf of Mexico Marine Bony Shorefishes
Old Dominion University ODU Digital Commons Biological Sciences Theses & Dissertations Biological Sciences Summer 2016 Hotspots, Extinction Risk and Conservation Priorities of Greater Caribbean and Gulf of Mexico Marine Bony Shorefishes Christi Linardich Old Dominion University, [email protected] Follow this and additional works at: https://digitalcommons.odu.edu/biology_etds Part of the Biodiversity Commons, Biology Commons, Environmental Health and Protection Commons, and the Marine Biology Commons Recommended Citation Linardich, Christi. "Hotspots, Extinction Risk and Conservation Priorities of Greater Caribbean and Gulf of Mexico Marine Bony Shorefishes" (2016). Master of Science (MS), Thesis, Biological Sciences, Old Dominion University, DOI: 10.25777/hydh-jp82 https://digitalcommons.odu.edu/biology_etds/13 This Thesis is brought to you for free and open access by the Biological Sciences at ODU Digital Commons. It has been accepted for inclusion in Biological Sciences Theses & Dissertations by an authorized administrator of ODU Digital Commons. For more information, please contact [email protected]. HOTSPOTS, EXTINCTION RISK AND CONSERVATION PRIORITIES OF GREATER CARIBBEAN AND GULF OF MEXICO MARINE BONY SHOREFISHES by Christi Linardich B.A. December 2006, Florida Gulf Coast University A Thesis Submitted to the Faculty of Old Dominion University in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE BIOLOGY OLD DOMINION UNIVERSITY August 2016 Approved by: Kent E. Carpenter (Advisor) Beth Polidoro (Member) Holly Gaff (Member) ABSTRACT HOTSPOTS, EXTINCTION RISK AND CONSERVATION PRIORITIES OF GREATER CARIBBEAN AND GULF OF MEXICO MARINE BONY SHOREFISHES Christi Linardich Old Dominion University, 2016 Advisor: Dr. Kent E. Carpenter Understanding the status of species is important for allocation of resources to redress biodiversity loss. -

Universidade Federal De Santa Catarina Centro De Ciências Biológicas Programa De Pós-Graduação Em Ecologia
UNIVERSIDADE FEDERAL DE SANTA CATARINA CENTRO DE CIÊNCIAS BIOLÓGICAS PROGRAMA DE PÓS-GRADUAÇÃO EM ECOLOGIA ANDREA DALBEN SOARES ESTRUTURA DE COMUNIDADE E DISTRIBUIÇÃO VERTICAL DE PEIXES CRIPTOBÊNTICOS EM ILHAS COSTEIRAS DE SANTA CATARINA, SUL DO BRASIL Florianópolis/SC 2010 ANDREA DALBEN SOARES ESTRUTURA DE COMUNIDADE E DISTRIBUIÇÃO VERTICAL DE PEIXES CRIPTOBÊNTICOS EM ILHAS COSTEIRAS DE SANTA CATARINA, SUL DO BRASIL Dissertação apresentada ao Programa de Pós-Graduação em Ecologia da Universidade Federal de Santa Catarina, para a obtenção do título de Mestre em Ecologia. Área de concentração : Ecologia, Bases Ecológicas para o Manejo e Conservação de Ecossistemas Costeiros. Orientador: Prof. Dr. Sergio R. Floeter Florianópolis/SC 2010 AGRADECIMENTOS À minha mãe e ao meu pai (in memorian) por me incentivarem e proporcionarem meios para que eu estudasse “aquilo que eu gostasse”, nunca pressionando para que essa escolha fosse direcionada a uma carreira “com um maior mercado de trabalho”, permitindo assim que hoje eu possa ter concluído um trabalho relacionado à ecologia de uns peixinhos pequenininhos que quase ninguém conhece! A Sérgio R. Floeter, por ter acreditado e confiado em mim desde o princípio de um projeto que mais parecia de doutorado (quem sabe agora!?). Agradeço também pela FORÇA nas correções e compreensão durante minhas viagens. Espero ter correspondido. Ao pessoal de campo que me ajudou muito mesmo quando a água estava 14°C: Anderson Batista, Ana Liedke, Igor Pinheiro, Fabrício Richmond. Aos membros da banca (Sonia Buck, Carlos Rangel e Barbara Segal) pelas sugestões e melhorias para este trabalho. À Cecilia Kotzias pelas correções do inglês, ao pessoal do LBMM (www.lbmm.ufsc.br) por todas as sugestões.