Factors Influencing Bee Communities and Pollination Services Across an Urban Environment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Reproductive Biology of Proboscidea Louisianica Is Investigated with Special Emphasis on the Insect-Plant Interrelationship
THE REPRODUCTIVE BIOLOGY OF PROBOSCIDEA LOUISIANICA (MARTYNIACEAE) by MARY ANN PHILLIPPI,, Bachelor of Science in Biological Science Auburn University Auburn, Alabama 1974 Submitted to the Faculty of the Graduate College of the Oklahoma State University in partial fulfillment of the requirements for the Degree of MASTER OF SCIENCE May' 1977 The.;s 1s /'177 P557r ~.;;.. THE REPRODUCTIVE BIOLOGY OF PROBOSCIDEA LOUISIANICA (MARTYNIACEAE) Thesis Approved: Dean of Graduate College ii PREFACE The reproductive biology of Proboscidea louisianica is investigated with special emphasis on the insect-plant interrelationship. This study included only one flowering season in only a small part of the plant's range. In order to more accurately elucidate the insect-plant interrelationship much more work is needed throughout Proboscidea louisianica's range. I wish to thank Dr. Ronald J. Tyrl, my thesis adviser, for his time and effort throughout my project. Appreciation is also extended to Dr. William A. Drew and Dr. James K. McPherson for advice and criticism throughout the course of this study and during the prepara tion of this manuscript. To Dr. Charles D. Michener, at the University of Kansas; Dr. H. E. Milliron, in New Martinsville, West Virginia; and Dr. T. B. Mitchell, at North Carolina State University I extend my appreciation for their time and expertise in identifying the insects collected during this study. Special thanks are given to Jim Petranka and to my family, Dr. and Mrs. G. M. Phillippi, Carolyn, Dan, and Jane for their encouragement in this and all endeavors. iii TABLE OF CONTENTS Page INTRODUCTION . 1 PHENOLOGY 6 INSECT VISITORS AND POLLINATION 10 THE SENSITIVE STIGMA . -

Gaddsteklar I Östergötland – Inventeringar I Sand- Och Grusmiljöer 2002-2007, Samt Övriga Fynd I Östergötlands Län
Gaddsteklar i Östergötland Inventeringar i sand- och grusmiljöer 2002-2007, samt övriga fynd i Östergötlands län LÄNSSTYRELSEN ÖSTERGÖTLAND Titel: Gaddsteklar i Östergötland – Inventeringar i sand- och grusmiljöer 2002-2007, samt övriga fynd i Östergötlands län Författare: Tommy Karlsson Utgiven av: Länsstyrelsen Östergötland Hemsida: http://www.e.lst.se Beställningsadress: Länsstyrelsen Östergötland 581 86 Linköping Länsstyrelsens rapport: 2008:9 ISBN: 978-91-7488-216-2 Upplaga: 400 ex Rapport bör citeras: Karlsson, T. 2008. Gaddsteklar i Östergötland – Inventeringar i sand- och grusmiljöer 2002-2007, samt övriga fynd i Östergötlands län. Länsstyrelsen Östergötland, rapport 2008:9. Omslagsbilder: Trätapetserarbi Megachile ligniseca Bålgeting Vespa crabro Finmovägstekel Arachnospila abnormis Illustrationer: Kenneth Claesson POSTADRESS: BESÖKSADRESS: TELEFON: TELEFAX: E-POST: WWW: 581 86 LINKÖPING Östgötagatan 3 013 – 19 60 00 013 – 10 31 18 [email protected] e.lst.se Rapport nr: 2008:9 ISBN: 978-91-7488-216-2 LÄNSSTYRELSEN ÖSTERGÖTLAND Förord Länsstyrelsen Östergötland arbetar konsekvent med för länet viktiga naturtyper inom naturvårdsarbetet. Med viktig menas i detta sammanhang biotoper/naturtyper som hyser en mångfald hotade arter och där Östergötland har ett stort ansvar – en stor andel av den svenska arealen och arterna. Det har tidigare inneburit stora satsningar på eklandskap, Omberg, skärgården, ängs- och hagmarker och våra kalkkärr och kalktorrängar. Till dessa naturtyper bör nu också de öppna sandmarkerna fogas. Denna inventering och sammanställning visar på dessa markers stora biologiska mångfald och rika innehåll av hotade och rödlistade arter. Detta är ju bra nog men dessutom betyder de solitära bina, humlorna och andra pollinerande insekter väldigt mycket för den ekologiska balansen och funktionaliteten i naturen. -
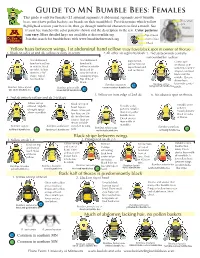
Guide to MN Bumble Bees: Females
Guide to MN Bumble Bees: Females This guide is only for females (12 antennal segments, 6 abdominal segments, most bumble Three small bees, most have pollen baskets, no beards on their mandibles). First determine which yellow eyes highlighted section your bee is in, then go through numbered characters to find a match. See if your bee matches the color patterns shown and the description in the text. Color patterns ® can vary. More detailed keys are available at discoverlife.org. Top of head Bee Front of face Squad Join the search for bumble bees with www.bumbleebeewatch.org Cheek Yellow hairs between wings, 1st abdominal band yellow (may have black spot in center of thorax) 1. Black on sides of 2nd ab, yellow or rusty in center 2.All other ab segments black 3. 2nd ab brownish centrally surrounded by yellow 2nd abdominal 2nd abdominal Light lemon Center spot band with yellow band with yellow hairs on on thorax with in middle, black yellow in middle top of head and sometimes faint V on sides. Yellow bordered by and on thorax. shaped extension often in a “W” rusty brown in a back from the shape. Top of swooping shape. middle. Queens head yellow. Top of head do not have black. Bombus impatiens Bombus affinis brownish central rusty patched bumble bee Bombus bimaculatus Bombus griseocollis common eastern bumble bee C patch. two-spotted bumble bee C brown-belted bumble bee C 5. Yellow on front edge of 2nd ab 6. No obvious spot on thorax. 4. 2nd ab entirely yellow and ab 3-6 black Yellow on top Black on top of Variable color of head. -

Las Abejas Del Género Agapostemon (Hymenoptera: Halictidae) Del Estado De Nuevo León, México
Revista Mexicana de Biodiversidad 83: 63-72, 2012 Las abejas del género Agapostemon (Hymenoptera: Halictidae) del estado de Nuevo León, México Bees of the genus Agapostemon (Hymenoptera: Halictidae) of the state of Nuevo León, Mexico Liliana Ramírez-Freire1 , Glafiro José Alanís-Flores1, Ricardo Ayala-Barajas2, Humberto Quiroz -Martínez1 y Carlos GerardoVelazco-Macías3 1Facultad de Ciencias Biológicas, Universidad Autónoma de Nuevo León, Cd. Universitaria. Apartado postal 134-F, 66450 San Nicolás de los Garza, Nuevo León, México. 2Estación de Biología Chamela (Sede Colima) Instituto de Biología, Universidad Nacional Autónoma de México. Apartado postal 21, 48980 San Patricio, Jalisco, México. 3Parques y Vida Silvestre. Av. Alfonso Reyes norte s/n, interior del Parque Niños Héroes, lateral izquierda, acceso 3, 64290 Monterrey, Nuevo León, México. [email protected] Resumen. Se realizó un estudio faunístico de las abejas del género Agapostemon (Halictidae) en el estado de Nuevo León, México para conocer las especies presentes, su distribución, relación con la flora y tipos de vegetación del estado. La metodología se basó en la revisión de literatura y de bases de datos de colecciones entomológicas, y en muestreos en campo donde se utilizó red entomológica y platos trampa de colores amarillo, azul, rosa (tonos fluorescentes) y blanco. Sólo en 20 de los 35 muestreos que se realizaron se obtuvieron ejemplares del género. Se recolectaron 11 especies, 2 de las cuales son registros nuevos para el estado (A. nasutus y A. splendens). El 12.31% de los ejemplares se obtuvo mediante el uso de red y el 87.84% con los platos trampa; el color amarillo fue el preferido por las abejas. -

UC Berkeley UC Berkeley Electronic Theses and Dissertations
UC Berkeley UC Berkeley Electronic Theses and Dissertations Title Bees and belonging: Pesticide detection for wild bees in California agriculture and sense of belonging for undergraduates in a mentorship program Permalink https://escholarship.org/uc/item/8z91q49s Author Ward, Laura True Publication Date 2020 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California Bees and belonging: Pesticide detection for wild bees in California agriculture and sense of belonging for undergraduates in a mentorship program By Laura T Ward A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Environmental Science, Policy, and Management in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Nicholas J. Mills, Chair Professor Erica Bree Rosenblum Professor Eileen A. Lacey Fall 2020 Bees and belonging: Pesticide detection for wild bees in California agriculture and sense of belonging for undergraduates in a mentorship program © 2020 by Laura T Ward Abstract Bees and belonging: Pesticide detection for wild bees in California agriculture and sense of belonging for undergraduates in a mentorship program By Laura T Ward Doctor of Philosophy in Environmental Science, Policy, and Management University of California, Berkeley Professor Nicholas J. Mills, Chair This dissertation combines two disparate subjects: bees and belonging. The first two chapters explore pesticide exposure for wild bees and honey bees visiting crop and non-crop plants in northern California agriculture. The final chapter utilizes surveys from a mentorship program as a case study to analyze sense of belonging among undergraduates. The first chapter of this dissertation explores pesticide exposure for wild bees and honey bees visiting sunflower crops. -

Bumble Bee Abundance in New York City Community Gardens: Implications for Urban Agriculture
Matteson and Langellotto: URBAN BUMBLE BEE ABUNDANCE Cities and the Environment 2009 Volume 2, Issue 1 Article 5 Bumble Bee Abundance in New York City Community Gardens: Implications for Urban Agriculture Kevin C. Matteson and Gail A. Langellotto Abstract A variety of crops are grown in New York City community gardens. Although the production of many crops benefits from pollination by bees, little is known about bee abundance in urban community gardens or which crops are specifically dependent on bee pollination. In 2005, we compiled a list of crop plants grown within 19 community gardens in New York City and classified these plants according to their dependence on bee pollination. In addition, using mark-recapture methods, we estimated the abundance of a potentially important pollinator within New York City urban gardens, the common eastern bumble bee (Bombus impatiens). This species is currently recognized as a valuable commercial pollinator of greenhouse crops. However, wild populations of B. impatiens are abundant throughout its range, including in New York City community gardens, where it is the most abundant native bee species present and where it has been observed visiting a variety of crop flowers. We conservatively counted 25 species of crop plants in 19 surveyed gardens. The literature suggests that 92% of these crops are dependent, to some degree, on bee pollination in order to set fruit or seed. Bombus impatiens workers were observed visiting flowers of 78% of these pollination-dependent crops. Estimates of the number of B. impatiens workers visiting individual gardens during the study period ranged from 3 to 15 bees per 100 m2 of total garden area and 6 to 29 bees per 100 m2 of garden floral area. -

Bumble Bees of CT-Females
Guide to CT Bumble Bees: Females This guide is only for females (12 antennal segments, 6 abdominal segments, most bumble bees, most have pollen baskets, no beards on their mandibles). First determine which yellow Three small eyes highlighted section your bee is in, then go through numbered characters to find a match. See if your bee matches the color patterns shown and the description in the text. Color patterns can vary. More detailed keys are available at discoverlife.org. Top of head Join the search for bumble bees with www.bumbleebeewatch.org Front of face Cheek Yellow hairs between wings, 1st abdominal band yellow (may have black spot in center of thorax) 1. Black on sides of 2nd ab, yellow or rusty in center 2.All other ab segments black 3. 2nd ab brownish centrally surrounded by yellow 2nd abdominal 2nd abdominal Light lemon Center spot band with yellow band with yellow hairs on on thorax with in middle, black yellow in middle top of head and sometimes faint V on sides. Yellow bordered by and on thorax. shaped extension often in a “W” rusty brown in a back from the shape. Top of swooping shape. middle. Queens head yellow. Top of head do not have black. Bombus impatiens Bombus affinis brownish central rusty patched bumble bee Bombus bimaculatus Bombus griseocollis common eastern bumble bee patch. two-spotted bumble bee brown-belted bumble bee 4. 2nd ab entirely yellow and ab 3-6 black 5. No obvious spot on thorax. Yellow on top Black on top of Variable color of head. -

List of Insect Species Which May Be Tallgrass Prairie Specialists
Conservation Biology Research Grants Program Division of Ecological Services © Minnesota Department of Natural Resources List of Insect Species which May Be Tallgrass Prairie Specialists Final Report to the USFWS Cooperating Agencies July 1, 1996 Catherine Reed Entomology Department 219 Hodson Hall University of Minnesota St. Paul MN 55108 phone 612-624-3423 e-mail [email protected] This study was funded in part by a grant from the USFWS and Cooperating Agencies. Table of Contents Summary.................................................................................................. 2 Introduction...............................................................................................2 Methods.....................................................................................................3 Results.....................................................................................................4 Discussion and Evaluation................................................................................................26 Recommendations....................................................................................29 References..............................................................................................33 Summary Approximately 728 insect and allied species and subspecies were considered to be possible prairie specialists based on any of the following criteria: defined as prairie specialists by authorities; required prairie plant species or genera as their adult or larval food; were obligate predators, parasites -

Wild Bee Declines and Changes in Plant-Pollinator Networks Over 125 Years Revealed Through Museum Collections
University of New Hampshire University of New Hampshire Scholars' Repository Master's Theses and Capstones Student Scholarship Spring 2018 WILD BEE DECLINES AND CHANGES IN PLANT-POLLINATOR NETWORKS OVER 125 YEARS REVEALED THROUGH MUSEUM COLLECTIONS Minna Mathiasson University of New Hampshire, Durham Follow this and additional works at: https://scholars.unh.edu/thesis Recommended Citation Mathiasson, Minna, "WILD BEE DECLINES AND CHANGES IN PLANT-POLLINATOR NETWORKS OVER 125 YEARS REVEALED THROUGH MUSEUM COLLECTIONS" (2018). Master's Theses and Capstones. 1192. https://scholars.unh.edu/thesis/1192 This Thesis is brought to you for free and open access by the Student Scholarship at University of New Hampshire Scholars' Repository. It has been accepted for inclusion in Master's Theses and Capstones by an authorized administrator of University of New Hampshire Scholars' Repository. For more information, please contact [email protected]. WILD BEE DECLINES AND CHANGES IN PLANT-POLLINATOR NETWORKS OVER 125 YEARS REVEALED THROUGH MUSEUM COLLECTIONS BY MINNA ELIZABETH MATHIASSON BS Botany, University of Maine, 2013 THESIS Submitted to the University of New Hampshire in Partial Fulfillment of the Requirements for the Degree of Master of Science in Biological Sciences: Integrative and Organismal Biology May, 2018 This thesis has been examined and approved in partial fulfillment of the requirements for the degree of Master of Science in Biological Sciences: Integrative and Organismal Biology by: Dr. Sandra M. Rehan, Assistant Professor of Biology Dr. Carrie Hall, Assistant Professor of Biology Dr. Janet Sullivan, Adjunct Associate Professor of Biology On April 18, 2018 Original approval signatures are on file with the University of New Hampshire Graduate School. -

The Lily Pad
The Lily Pad certain flower seeds because of the July Program shape of their beak. Eleanor C. Foerste, Faculty, Natural They also found this was true of the July 2013 Resources, UF/IFAS Osceola County squirrels and the mice we saw. Volume 7, Issue 5 Extension will present on Invasive species - Air potato and the One young boy just could not stop biocontrol air potato beetle as a himself from reaching over to collect management tool. a few seeds for himself to take home to his own garden! His chosen plant? In the Community Dune sunflower. A native plant by Jenny Welch member in the making. The purpose of the Florida Native Plant Interesting that our class was about Society is to promote the preservation, Sandy Webb and I were asked to birds yet it still came back around to conservation, and restoration of the native help out at Bok Tower Summer native plants… plants and native plant communities of Camp Program. We were there for Florida. "Bountiful Birds" program. As I always say you cannot have BOARD OF DIRECTORS : birds without native plants and you President: cannot have native plants without Jenny Welch.............. [email protected] birds. We discussed what birds eat 1st Vice President: based upon their beaks. Mark Johnson ....... [email protected] We went on a walk to the Window Secretary: by the pond, a great place to see birds Sandy Webb....... [email protected] because it is a room with glass Treasurer: overlooking a small pond. OPEN ................................... Apply now Along the way we saw several Chapter Rep: birds…mockingbird, cardinal, blue ............................................. -

Creating a Pollinator Garden for Native Specialist Bees of New York and the Northeast
Creating a pollinator garden for native specialist bees of New York and the Northeast Maria van Dyke Kristine Boys Rosemarie Parker Robert Wesley Bryan Danforth From Cover Photo: Additional species not readily visible in photo - Baptisia australis, Cornus sp., Heuchera americana, Monarda didyma, Phlox carolina, Solidago nemoralis, Solidago sempervirens, Symphyotrichum pilosum var. pringlii. These shade-loving species are in a nearby bed. Acknowledgements This project was supported by the NYS Natural Heritage Program under the NYS Pollinator Protection Plan and Environmental Protection Fund. In addition, we offer our appreciation to Jarrod Fowler for his research into compiling lists of specialist bees and their host plants in the eastern United States. Creating a Pollinator Garden for Specialist Bees in New York Table of Contents Introduction _________________________________________________________________________ 1 Native bees and plants _________________________________________________________________ 3 Nesting Resources ____________________________________________________________________ 3 Planning your garden __________________________________________________________________ 4 Site assessment and planning: ____________________________________________________ 5 Site preparation: _______________________________________________________________ 5 Design: _______________________________________________________________________ 6 Soil: _________________________________________________________________________ 6 Sun Exposure: _________________________________________________________________ -

Unique Bee Communities Within Vacant Lots and Urban Farms Result from Variation in Surrounding Urbanization Intensity
sustainability Article Unique Bee Communities within Vacant Lots and Urban Farms Result from Variation in Surrounding Urbanization Intensity Frances S. Sivakoff ID , Scott P. Prajzner and Mary M. Gardiner * ID Department of Entomology, The Ohio State University, 2021 Coffey Road, Columbus, OH 43210, USA; [email protected] (F.S.S.); [email protected] (S.P.P.) * Correspondence: [email protected]; Tel.: +1-330-601-6628 Received: 1 May 2018; Accepted: 5 June 2018; Published: 8 June 2018 Abstract: We investigated the relative importance of vacant lot and urban farm habitat features and their surrounding landscape context on bee community richness, abundance, composition, and resource use patterns. Three years of pan trap collections from 16 sites yielded a rich assemblage of bees from vacant lots and urban farms, with 98 species documented. We collected a greater bee abundance from vacant lots, and the two forms of greenspace supported significantly different bee communities. Plant–pollinator networks constructed from floral visitation observations revealed that, while the average number of bees utilizing available resources, niche breadth, and niche overlap were similar, the composition of floral resources and common foragers varied by habitat type. Finally, we found that the proportion of impervious surface and number of greenspace patches in the surrounding landscape strongly influenced bee assemblages. At a local scale (100 m radius), patch isolation appeared to limit colonization of vacant lots and urban farms. However, at a larger landscape scale (1000 m radius), increasing urbanization resulted in a greater concentration of bees utilizing vacant lots and urban farms, illustrating that maintaining greenspaces provides important habitat, even within highly developed landscapes.