PETITION to LIST the VARIABLE CUCKOO BUMBLE BEE Bombus Variabilis (Cresson 1872) UNDER the ENDANGERED SPECIES ACT and CONCURRENTLY DESIGNATE CRITICAL HABITAT
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Wisconsin Bee Identification Guide
WisconsinWisconsin BeeBee IdentificationIdentification GuideGuide Developed by Patrick Liesch, Christy Stewart, and Christine Wen Honey Bee (Apis mellifera) The honey bee is perhaps our best-known pollinator. Honey bees are not native to North America and were brought over with early settlers. Honey bees are mid-sized bees (~ ½ inch long) and have brownish bodies with bands of pale hairs on the abdomen. Honey bees are unique with their social behavior, living together year-round as a colony consisting of thousands of individuals. Honey bees forage on a wide variety of plants and their colonies can be useful in agricultural settings for their pollination services. Honey bees are our only bee that produces honey, which they use as a food source for the colony during the winter months. In many cases, the honey bees you encounter may be from a local beekeeper’s hive. Occasionally, wild honey bee colonies can become established in cavities in hollow trees and similar settings. Photo by Christy Stewart Bumble bees (Bombus sp.) Bumble bees are some of our most recognizable bees. They are amongst our largest bees and can be close to 1 inch long, although many species are between ½ inch and ¾ inch long. There are ~20 species of bumble bees in Wisconsin and most have a robust, fuzzy appearance. Bumble bees tend to be very hairy and have black bodies with patches of yellow or orange depending on the species. Bumble bees are a type of social bee Bombus rufocinctus and live in small colonies consisting of dozens to a few hundred workers. Photo by Christy Stewart Their nests tend to be constructed in preexisting underground cavities, such as former chipmunk or rabbit burrows. -

Seasonal and Spatial Patterns of Mortality and Sex Ratio in the Alfalfa
Seasonal and spatial patterns of mortality and sex ratio in the alfalfa leafcutting bee, Megachile rotundata (F.) by Ruth Pettinga ONeil A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Entomology Montana State University © Copyright by Ruth Pettinga ONeil (2004) Abstract: Nests from five seed alfalfa sites of the alfalfa leafcutting bee Megachile rotundata (F.) were monitored over the duration of the nesting season in 2000 and 2001, from early July through late August. Cells containing progeny of known age and known position within the nest were subsequently analyzed for five commonly encountered categories of pre-diapause mortality in this species. Chalkbrood and pollen ball had the strongest seasonal relationships of mortality factors studied. Chalkbrood incidence was highest in early-produced cells. Pollen ball was higher in late-season cells. Chalkbrood, parasitism by the chalcid Pteromalus venustus, and death of older larvae and prepupae , due to unknown source(s) exhibited the strongest cell-position relationships. Both chalkbrood and parasitoid incidence were highest in the inner portions of nests. The “unknown” category of mortality was highest in outer portions of nests. Sex ratio was determined for a subset of progeny reared to adulthood. The ratio of females to males is highest in cells in inner nest positions. Sex ratio is female-biased very early in the nesting season, when all cells being provisioned are the inner cells of nests, due to the strong positional effect on sex ratio. SEASONAL AND SPATIAL PATTERNS OF MORTALITY AND SEX RATIO IN THE ALFALFA LEAFCUTTING BEE, Megachile rotundata (F.) by . -
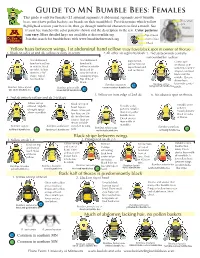
Guide to MN Bumble Bees: Females
Guide to MN Bumble Bees: Females This guide is only for females (12 antennal segments, 6 abdominal segments, most bumble Three small bees, most have pollen baskets, no beards on their mandibles). First determine which yellow eyes highlighted section your bee is in, then go through numbered characters to find a match. See if your bee matches the color patterns shown and the description in the text. Color patterns ® can vary. More detailed keys are available at discoverlife.org. Top of head Bee Front of face Squad Join the search for bumble bees with www.bumbleebeewatch.org Cheek Yellow hairs between wings, 1st abdominal band yellow (may have black spot in center of thorax) 1. Black on sides of 2nd ab, yellow or rusty in center 2.All other ab segments black 3. 2nd ab brownish centrally surrounded by yellow 2nd abdominal 2nd abdominal Light lemon Center spot band with yellow band with yellow hairs on on thorax with in middle, black yellow in middle top of head and sometimes faint V on sides. Yellow bordered by and on thorax. shaped extension often in a “W” rusty brown in a back from the shape. Top of swooping shape. middle. Queens head yellow. Top of head do not have black. Bombus impatiens Bombus affinis brownish central rusty patched bumble bee Bombus bimaculatus Bombus griseocollis common eastern bumble bee C patch. two-spotted bumble bee C brown-belted bumble bee C 5. Yellow on front edge of 2nd ab 6. No obvious spot on thorax. 4. 2nd ab entirely yellow and ab 3-6 black Yellow on top Black on top of Variable color of head. -

AUG-2017-ACB-Newslet
Newsletter for August 2017 Monthly Meeting Equipment Available Saturday, August 19th, 3:00 p.m. Don Moore has slowly scaled back his number of Hive Work and hives and equipment over the last few Ice Cream Social @ years. He plans to reduce his hives by another 9 Breezy Acres this year, leaving him with 5 hives to manage. He will offer those 9 hives for sale at the August meeting for $150 each. Each hive consists of a solid 3634 Stoney Creek Church Road bottom board, two 10-frame deep supers, a screen Elon, NC 27244 inner cover, a telescoping lid and a full staff of hon- ey bees. Queen excluders are not on the hives, but Don and Shirley Moore welcome us to their will be provided when you pick up the bees. apiary for some up-close reviewing and Other equipment will also be offered for sale on learning. We’ll spend about an hour and a meeting day (8/19) and will be appropriately half opening up hives and seeing what’s priced. These include hive top feeders, division going on inside, and we’ll talk about re- board feeders, excluders, spacers, honey supers queening and other hive work for the sea- with drawn comb, etc. The equipment is used, but son. Nancy Ruppert and Don Hopkins will in serviceable condition. The price of new wooden- be our excellent guides. ware for a hive as described is more than the $150 price advertised. Then we’ll make our way to the shade and FOR SALE: enjoy some home- made ice cream and 4 complete hives with bees. -

Bumble Bees of CT-Females
Guide to CT Bumble Bees: Females This guide is only for females (12 antennal segments, 6 abdominal segments, most bumble bees, most have pollen baskets, no beards on their mandibles). First determine which yellow Three small eyes highlighted section your bee is in, then go through numbered characters to find a match. See if your bee matches the color patterns shown and the description in the text. Color patterns can vary. More detailed keys are available at discoverlife.org. Top of head Join the search for bumble bees with www.bumbleebeewatch.org Front of face Cheek Yellow hairs between wings, 1st abdominal band yellow (may have black spot in center of thorax) 1. Black on sides of 2nd ab, yellow or rusty in center 2.All other ab segments black 3. 2nd ab brownish centrally surrounded by yellow 2nd abdominal 2nd abdominal Light lemon Center spot band with yellow band with yellow hairs on on thorax with in middle, black yellow in middle top of head and sometimes faint V on sides. Yellow bordered by and on thorax. shaped extension often in a “W” rusty brown in a back from the shape. Top of swooping shape. middle. Queens head yellow. Top of head do not have black. Bombus impatiens Bombus affinis brownish central rusty patched bumble bee Bombus bimaculatus Bombus griseocollis common eastern bumble bee patch. two-spotted bumble bee brown-belted bumble bee 4. 2nd ab entirely yellow and ab 3-6 black 5. No obvious spot on thorax. Yellow on top Black on top of Variable color of head. -
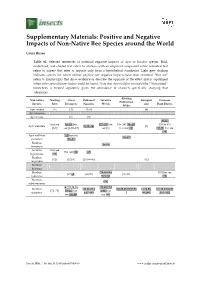
Positive and Negative Impacts of Non-Native Bee Species Around the World
Supplementary Materials: Positive and Negative Impacts of Non-Native Bee Species around the World Laura Russo Table S1. Selected references of potential negative impacts of Apis or Bombus species. Bold, underlined, and shaded text refers to citations with an empirical component while unbolded text refers to papers that refer to impacts only from a hypothetical standpoint. Light grey shading indicates species for which neither positive nor negative impacts have been recorded. “But see” refers to manuscripts that show evidence or describe the opposite of the effect and is capitalized when only contradictory studies could be found. Note that Apis mellifera scutellata (the “Africanized” honeybee), is treated separately given the abundance of research specifically studying that subspecies. Altering Non-native Nesting Floral Pathoens/ Invasive Introgres Decrease Pollination Species Sites Resources Parasites Weeds sion Plant Fitness Webs Apis cerana [1] [2] [1–3] [4] Apis dorsata Apis florea [5] [5] [37,45] But see [8–19] but [27–35] but [36–38] [39–43] [38,46,47] Apis mellifera [9,23–26] [4] [6,7] see [6,20–22] see [6] but see [44] [48,49] but see [50] Apis mellifera [51] but see [55–57] scutellata [52–54] Bombus [58,59] hortorum Bombus But see But see [60] [61] hypnorum [60] Bombus [62] [62,63] [26,64–66] [62] impatiens Bombus lucorum Bombus [28,58,59,6 [39] but see [67,68] [69,70] [36,39] ruderatus 9,71,72] [73] Bombus [59] subterraneous [67,70,74,75, [29,58,72,9 Bombus [25,26,70,7 [38,39,68,81,97,98 [4,76,88, [47,76,49,86,97 [74–76] 77–84] but 1–95] but terrestris 6,87–90] ] 99,100] ,101–103] see [85,86] see [96] Insects 2016, 7, 69; doi:10.3390/insects7040069 www.mdpi.com/journal/insects Insects 2016, 7, 69 S2 of S8 Table S2. -

Bees and Wasps of the East Sussex South Downs
A SURVEY OF THE BEES AND WASPS OF FIFTEEN CHALK GRASSLAND AND CHALK HEATH SITES WITHIN THE EAST SUSSEX SOUTH DOWNS Steven Falk, 2011 A SURVEY OF THE BEES AND WASPS OF FIFTEEN CHALK GRASSLAND AND CHALK HEATH SITES WITHIN THE EAST SUSSEX SOUTH DOWNS Steven Falk, 2011 Abstract For six years between 2003 and 2008, over 100 site visits were made to fifteen chalk grassland and chalk heath sites within the South Downs of Vice-county 14 (East Sussex). This produced a list of 227 bee and wasp species and revealed the comparative frequency of different species, the comparative richness of different sites and provided a basic insight into how many of the species interact with the South Downs at a site and landscape level. The study revealed that, in addition to the character of the semi-natural grasslands present, the bee and wasp fauna is also influenced by the more intensively-managed agricultural landscapes of the Downs, with many species taking advantage of blossoming hedge shrubs, flowery fallow fields, flowery arable field margins, flowering crops such as Rape, plus plants such as buttercups, thistles and dandelions within relatively improved pasture. Some very rare species were encountered, notably the bee Halictus eurygnathus Blüthgen which had not been seen in Britain since 1946. This was eventually recorded at seven sites and was associated with an abundance of Greater Knapweed. The very rare bees Anthophora retusa (Linnaeus) and Andrena niveata Friese were also observed foraging on several dates during their flight periods, providing a better insight into their ecology and conservation requirements. -

Medical Entomology in Brief
Medical Entomology in Brief Dr. Alfatih Saifudinn Aljafari Assistant professor of Parasitology College of Medicine- Al Jouf University Aim and objectives • Aim: – To bring attention to medical entomology as important biomedical science • Objective: – By the end of this presentation, audience could be able to: • Understand the scope of Medical Entomology • Know medically important arthropods • Understand the basic of pathogen transmission dynamic • Medical Entomology in Brief- Dr. Aljafari (CME- January 2019) In this presentation • Introduction • Classification of arthropods • Examples of medical and public health important species • Insect Ethology • Dynamic of disease transmission • Other application of entomology Medical Entomology in Brief- Dr. Aljafari (CME- January 2019) Definition • Entomology: – The branch of zoology concerned with the study of insects. • Medical Entomology: – Branch of Biomedical sciences concerned with “ArthrobodsIn the past the term "insect" was more vague, and historically the definition of entomology included the study of terrestrial animals in other arthropod groups or other phyla, such, as arachnids, myriapods, earthworms, land snails, and slugs. This wider meaning may still be encountered in informal use. • At some 1.3 million described species, insects account for more than two-thirds of all known organisms, date back some 400 million years, and have many kinds of interactions with humans and other forms of life on earth Medical Entomology in Brief- Dr. Aljafari (CME- January 2019) Arthropods and Human • Transmission of infectious agents • Allergy • Injury • Inflammation • Agricultural damage • Termites • Honey • Silk Medical Entomology in Brief- Dr. Aljafari (CME- January 2019) Phylum Arthropods • Hard exoskeleton, segmented bodies, jointed appendages • Nearly one million species identified so far, mostly insects • The exoskeleton, or cuticle, is composed of chitin. -

Assessing Bumble Bee Diversity, Distribution, and Status for the Michigan Wildlife Action Plan
Assessing Bumble Bee Diversity, Distribution, and Status for the Michigan Wildlife Action Plan Prepared By: Logan M. Rowe, David L. Cuthrell, and Helen D. Enander Michigan Natural Features Inventory Michigan State University Extension P.O. Box 13036 Lansing, MI 48901 Prepared For: Michigan Department of Natural Resources Wildlife Division 12/17/2019 MNFI Report No. 2019-33 Suggested Citation: Rowe, L. M., D. L. Cuthrell., H. D. Enander. 2019. Assessing Bumble Bee Diversity, Distribution, and Status for the Michigan Wildlife Action Plan. Michigan Natural Features Inventory, Report Number 2019- 33, Lansing, USA. Copyright 2019 Michigan State University Board of Trustees. MSU Extension programs and materials are open to all without regard to race, color, national origin, gender, religion, age, disability, political beliefs, sexual orientation, marital status or family status. Cover: Bombus terricola taken by D. L. Cuthrell Table of Contents Abstract ........................................................................................................................................................ iii Introduction .................................................................................................................................................. 1 Methods ........................................................................................................................................................ 2 Museum Searches .................................................................................................................................... -

Bees, Wasps & Ants
Sheringham and Beeston Regis Commons SSSI / SAC FAUNA: Hymenoptera INSECTA (Pterygota) Family/Order English Name. Scientific Name. Authority. Grid Ref. Tetrad/ Last Km sq. Common. Record. HYMENOPTERA. PAMPHILIDAE: Sawfly. Pamphilius inanitus (Villers, 1789) TG1642 1987? (Bees, Wasps and Ants) ARGIDAE: Elm Zig-zag Sawfly. Aproceros leucopoda Takeuchi, 1939 TG1642 14R/B 2020 Bramble Sawfly. Arge cyaneocrocea (Forster, 1771) TG1642 2016 Sawfly. Arge gracilicornis (Klug, 1814 ) TG1642 1987? CIMBICIDAE: Honeysuckle Sawfly. Abia lonicerae (Linnaeus) TG1641 14Q/B 2015 Club-horned Sawfly. Abia sericera (Linnaeus) TG1642 14R/B 2014 Club-horned Sawfly. Zaraea fasciata Linnaeus, 1758 TG1641/42 14R,14Q/B 2014 Birch Sawfly. Cimbex femoratus (Linnaeus, 1758) TG1642 14R/B 2017 SIRICIDAE: Greater Horntail Wasp. Urocerus gigas (Linnaeus, 1758) TG1642 14R/S 1992 CEPHIDAE: Sawfly. Calameuta pallipes (Klug, 1803) TG1642 1987? TENTHREDINIDAE: Willow Sawfly. Pontania proxima (Lepeletier, 1823) TG1642 14R/BS 2009 Willow Sawfly. Eupontania pedunculi (Hartig, 1837) TG1642 14R/B 1999 Willow Sawfly. Eupontainia viminalis (Linnaeus, 1758) TG1642 14R/B 2002 Willow Sawfly. Pontainia bridgemanii (Cameron, 1883) TG1642 14R/B 1999 Sawfly. Caliroa annulipes (Klug, 1816) TG1642 14R/S 2002 Hazel Sawfly. Craesus septentrionalis (Linnaeus, 1758) TG1641 14Q/B 2017 Sawfly. Blennocampa phyllocolpa Viitasaari & Vikberg, 1985 TG1642/41 14R,14Q/B 2003 Sawfly. Selandria serva (Fabricius, 1793) TG1642 14R/B 2013 Sawfly. Aneugmenus padi (Linnaeus, 1761) TG1642 1987? Bracken Sawfly. Strongylogaster multifasciata (Geoffroy, 1785) TG1642 14R/BS 2020 Sawfly. Dichrodolerus vestigialis (Klug, 1818) TG1642 1996 Sawfly. Dolerus germanicus (Fabricius, 1775) TG1642 1987? Sawfly. Eutomostethus ephippium (Panzer, 1798) TG1642 14R/BS 2020 Sawfly. Poodolerus aeneus Hartig, 1837 TG1642 1987? Sawfly. Dolerus brevitarus Hartig TG1642 1987? Sawfly. -

Beyond the Decline of Wild Bees: Optimizing Conservation Measures and Bringing Together the Actors
insects Review Beyond the Decline of Wild Bees: Optimizing Conservation Measures and Bringing Together the Actors Maxime Drossart * and Maxence Gérard * Laboratory of Zoology, Research Institute for Biosciences, University of Mons (UMONS), Place du Parc 20, B-7000 Mons, Belgium * Correspondence: [email protected] (M.D.); [email protected] (M.G.) Received: 3 September 2020; Accepted: 18 September 2020; Published: 22 September 2020 Simple Summary: Wild bees represent the main group of pollinators in Europe, being responsible for the reproduction of numerous flowering plants. However, like a non-negligible part of biodiversity, this group has been facing a global decline mostly induced by numerous human factors over the last decades. Overall, even if all the questions are not solved concerning the causes of their decline, we are beyond the precautionary principle because the decline factors are roughly known, identified and at least partially quantified. Experts are now calling for effective actions to promote wild bee diversity and the enhancement of environmental quality. In this review, we present a general and up-to-date assessment of the conservation methods, as well as their efficiency and the current projects that try to fill the gaps and optimize the conservation measures. This publication aims to be a needed catalyst to implement concrete and qualitative conservation actions for wild bees. Abstract: Wild bees are facing a global decline mostly induced by numerous human factors for the last decades. In parallel, public interest for their conservation increased considerably, namely through numerous scientific studies relayed in the media. In spite of this broad interest, a lack of knowledge and understanding of the subject is blatant and reveals a gap between awareness and understanding. -

Bumblebee Conservator
Volume 2, Issue 1: First Half 2014 Bumblebee Conservator Newsletter of the BumbleBee Specialist Group In this issue From the Chair From the Chair 1 A very happy and productive 2014 to everyone! We start this year having seen From the Editor 1 enormously encouraging progress in 2013. Our different regions have started from BBSG Executive Committee 2 very different positions, in terms of established knowledge of their bee faunas Regional Coordinators 2 as well as in terms of resources available, but members in all regions are actively moving forward. In Europe and North America, which have been fortunate to Bumblebee Specialist have the most specialists over the last century, we are achieving the first species Group Report 2013 3 assessments. Mesoamerica and South America are also very close, despite the huge Bumblebees in the News 9 areas to survey and the much less well known species. In Asia, with far more species, many of them poorly known, remarkably rapid progress is being made in sorting Research 13 out what is present and in building the crucial keys and distribution maps. In some Conservation News 20 regions there are very few people to tackle the task, sometimes in situations that Bibliography 21 make progress challenging and slow – their enthusiasm is especially appreciated! At this stage, broad discussion of problems and of the solutions developed from your experience will be especially important. This will direct the best assessments for focusing the future of bumblebee conservation. From the Editor Welcome to the second issue of the Bumblebee Conservator, the official newsletter of the Bumblebee Specialist Group.