Assessing Bumble Bee Diversity, Distribution, and Status for the Michigan Wildlife Action Plan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bumble Bees of the Western United States” by Jonathan Koch, James Strange, and Paul Williams (2012)
Adapted from the “Bumble Bees of the Western United States” by Jonathan Koch, James Strange, and Paul Williams (2012). Bumble bees are one of Wyoming’s most important and mild-mannered pollinators. There are more than 20 species in Wyoming, which you can often tell apart by the color patterns on their bodies. This guide shows color patterns for queen bees only, and some species can have multiple queen Bumble patterns! of Bees Areas in yellow indicate where each species Wyoming is found in Wyoming wyomingbiodiversity.org Black Tail Bumble Bee How can you tell Bombus melanopygus it's a bumble bee? Common Bumble bees are the largest bodied bees in Wyoming. Queens can be up to two inches long, but most queens and workers are somewhat smaller than that. They're very hairy all over their bodies, and carry pollen in “baskets” on their hind legs. Did you find a bumble bee? Submit your observation to the Xerces Society of Invertebrate Conservation's website: BumbleBeeWatch.org You can be a member of the citizen science community! Brown-Belted Bumble Bee California Bumble Bee Bombus griseocollis Bombus californicus Common Uncommon Central Bumble Bee Cuckoo Bumble Bee Bombus centralis Bombus insularis Common Common Fernald Cuckoo Bumble Bee Forest Bumble Bee Bombus fernaldae Bombus sylvicola Uncommon Uncommon Frigid Bumble Bee Fuzzy-Horned Bumble Bee Bombus frigidus Bombus mixtus Rare Common Half-Black Bumble Bee High Country Bumble Bee Bombus vagans Bombus balteatus Common Common Hunt’s Bumble Bee Morrison Bumble Bee Bombus huntii Bombus morrisoni Common Common Nevada Bumble Bee Red-Belted Bumble Bee Bombus nevadensis Bombus rufocinctus Common Common Suckley Cuckoo Bumble Bee Bombus suckleyi Red-Belted Bumble Bee, continued Uncommon Two-Form Bumble Bee Western Bumble Bee Bombus bifarius Bombus occidentalis Common Rare throughout much of its range, but common in Wyoming. -

The Maryland Entomologist
THE MARYLAND ENTOMOLOGIST Insect and related-arthropod studies in the Mid-Atlantic region Volume 7, Number 2 September 2018 September 2018 The Maryland Entomologist Volume 7, Number 2 MARYLAND ENTOMOLOGICAL SOCIETY www.mdentsoc.org Executive Committee: President Frederick Paras Vice President Philip J. Kean Secretary Janet A. Lydon Treasurer Edgar A. Cohen, Jr. Historian (vacant) Journal Editor Eugene J. Scarpulla E-newsletter Editors Aditi Dubey The Maryland Entomological Society (MES) was founded in November 1971, to promote the science of entomology in all its sub-disciplines; to provide a common meeting venue for professional and amateur entomologists residing in Maryland, the District of Columbia, and nearby areas; to issue a periodical and other publications dealing with entomology; and to facilitate the exchange of ideas and information through its meetings and publications. The MES was incorporated in April 1982 and is a 501(c)(3) non-profit, scientific organization. The MES logo features an illustration of Euphydryas phaëton (Drury) (Lepidoptera: Nymphalidae), the Baltimore Checkerspot, with its generic name above and its specific epithet below (both in capital letters), all on a pale green field; all these are within a yellow ring double-bordered by red, bearing the message “● Maryland Entomological Society ● 1971 ●”. All of this is positioned above the Shield of the State of Maryland. In 1973, the Baltimore Checkerspot was named the official insect of the State of Maryland through the efforts of many MES members. Membership in the MES is open to all persons interested in the study of entomology. All members receive the annual journal, The Maryland Entomologist, and the monthly e-newsletter, Phaëton. -

List of Animal Species with Ranks October 2017
Washington Natural Heritage Program List of Animal Species with Ranks October 2017 The following list of animals known from Washington is complete for resident and transient vertebrates and several groups of invertebrates, including odonates, branchipods, tiger beetles, butterflies, gastropods, freshwater bivalves and bumble bees. Some species from other groups are included, especially where there are conservation concerns. Among these are the Palouse giant earthworm, a few moths and some of our mayflies and grasshoppers. Currently 857 vertebrate and 1,100 invertebrate taxa are included. Conservation status, in the form of range-wide, national and state ranks are assigned to each taxon. Information on species range and distribution, number of individuals, population trends and threats is collected into a ranking form, analyzed, and used to assign ranks. Ranks are updated periodically, as new information is collected. We welcome new information for any species on our list. Common Name Scientific Name Class Global Rank State Rank State Status Federal Status Northwestern Salamander Ambystoma gracile Amphibia G5 S5 Long-toed Salamander Ambystoma macrodactylum Amphibia G5 S5 Tiger Salamander Ambystoma tigrinum Amphibia G5 S3 Ensatina Ensatina eschscholtzii Amphibia G5 S5 Dunn's Salamander Plethodon dunni Amphibia G4 S3 C Larch Mountain Salamander Plethodon larselli Amphibia G3 S3 S Van Dyke's Salamander Plethodon vandykei Amphibia G3 S3 C Western Red-backed Salamander Plethodon vehiculum Amphibia G5 S5 Rough-skinned Newt Taricha granulosa -
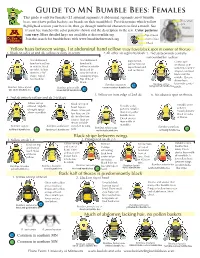
Guide to MN Bumble Bees: Females
Guide to MN Bumble Bees: Females This guide is only for females (12 antennal segments, 6 abdominal segments, most bumble Three small bees, most have pollen baskets, no beards on their mandibles). First determine which yellow eyes highlighted section your bee is in, then go through numbered characters to find a match. See if your bee matches the color patterns shown and the description in the text. Color patterns ® can vary. More detailed keys are available at discoverlife.org. Top of head Bee Front of face Squad Join the search for bumble bees with www.bumbleebeewatch.org Cheek Yellow hairs between wings, 1st abdominal band yellow (may have black spot in center of thorax) 1. Black on sides of 2nd ab, yellow or rusty in center 2.All other ab segments black 3. 2nd ab brownish centrally surrounded by yellow 2nd abdominal 2nd abdominal Light lemon Center spot band with yellow band with yellow hairs on on thorax with in middle, black yellow in middle top of head and sometimes faint V on sides. Yellow bordered by and on thorax. shaped extension often in a “W” rusty brown in a back from the shape. Top of swooping shape. middle. Queens head yellow. Top of head do not have black. Bombus impatiens Bombus affinis brownish central rusty patched bumble bee Bombus bimaculatus Bombus griseocollis common eastern bumble bee C patch. two-spotted bumble bee C brown-belted bumble bee C 5. Yellow on front edge of 2nd ab 6. No obvious spot on thorax. 4. 2nd ab entirely yellow and ab 3-6 black Yellow on top Black on top of Variable color of head. -

CUTOVERS AS POTENTIAL SUITABLE BEE HABITATS By
CUTOVERS AS POTENTIAL SUITABLE BEE HABITATS by Jasmine R. Pinksen A Thesis submitted in partial fulfillment of the requirements for the degree of Bachelor of Science, Honours Environmental Science, Grenfell Campus Memorial University of Newfoundland April 2017 Corner Brook Newfoundland 2 Grenfell Campus Environmental Science Unit The undersigned certify that they have read, and recommend to the Environmental Science Unit (School of Science and the Environment) for acceptance, a thesis entitled “Cutovers as Potential Suitable Bee Habitats” submitted by Jasmine R. Pinksen in partial fulfillment of the requirements for the degree of Bachelor of Science, Honours. _____________________________ Dr. Julie Sircom (thesis supervisor) _____________________________ Dr. Erin Fraser _____________________________ Dr. Joe Bowden April ____, 2017 3 Acknowledgements Thank you to the valuable research team members who helped with data collection for this project, Geena Arul Jothi, Megan Trotman, Tiffany Fillier, Erika Young, Nicole Walsh, and Abira Mumtaz. Thank you to Barry Elkins with Corner Brook Pulp and Paper Ltd. for financial support in 2015, maps of the logging areas around Corner Brook, NL, and his recommendations on site selection. Special thank you to my supervisor Dr. Julie Sircom, Dr. Erin Fraser and Dr. Joe Bowden for their feedback and support. 4 Abstract Bees are of great importance for pollinating agricultural crops and wild plant communities. Direct human activities such as urbanization, pesticide use, pollution, and introduction of species and pathogens as well as climate change are resulting in habitat loss and fragmentation for bees, causing declines in bee populations worldwide. Lack of suitable habitat is considered to be one of the main factors contributing to these declines. -

The Conservation Management and Ecology of Northeastern North
THE CONSERVATION MANAGEMENT AND ECOLOGY OF NORTHEASTERN NORTH AMERICAN BUMBLE BEES AMANDA LICZNER A DISSERTATION SUBMITTED TO THE FACULTY OF GRADUATE STUDIES IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY GRADUATE PROGRAM IN BIOLOGY YORK UNIVERSITY TORONTO, ONTARIO September 2020 © Amanda Liczner, 2020 ii Abstract Bumble bees (Bombus spp.; Apidae) are among the pollinators most in decline globally with a main cause being habitat loss. Habitat requirements for bumble bees are poorly understood presenting a research gap. The purpose of my dissertation is to characterize the habitat of bumble bees at different spatial scales using: a systematic literature review of bumble bee nesting and overwintering habitat globally (Chapter 1); surveys of local and landcover variables for two at-risk bumble bee species (Bombus terricola, and B. pensylvanicus) in southern Ontario (Chapter 2); identification of conservation priority areas for bumble bee species in Canada (Chapter 3); and an analysis of the methodology for locating bumble bee nests using detection dogs (Chapter 4). The main findings were current literature on bumble bee nesting and overwintering habitat is limited and biased towards the United Kingdom and agricultural habitats (Ch.1). Bumble bees overwinter underground, often on shaded banks or near trees. Nests were mostly underground and found in many landscapes (Ch.1). B. terricola and B. pensylvanicus have distinct habitat characteristics (Ch.2). Landscape predictors explained more variation in the species data than local or floral resources (Ch.2). Among local variables, floral resources were consistently important throughout the season (Ch.2). Most bumble bee conservation priority areas are in western Canada, southern Ontario, southern Quebec and across the Maritimes and are most often located within woody savannas (Ch.3). -

The Rusty Patched Bumble Bee (Bombus Affinis) Voluntary Implementation Guidance for Section 10(A)(1)(B) of the Endangered Species Act
The Rusty Patched Bumble Bee (Bombus affinis) Voluntary Implementation Guidance for Section 10(a)(1)(B) of the Endangered Species Act Version 1.1 U.S. Fish & Wildlife Service, Regions 3, 4, 5 and 6 March 21, 2017 Contents Background and Purpose ................................................................................................................ 1 Current Versions of this Guidance .................................................................................................. 1 Range of Rusty Patched Bumble Bee .............................................................................................. 1 Brief Description of the Habitat Model ...................................................................................... 2 Section 10(a)(1)(B) of the Endangered Species Act and the Rusty Patched Bumble Bee .............. 5 Screening and Evaluation of Projects – A Stepwise Approach ................................................... 5 Step 1. Determine whether the rusty patched bumble bee is likely to be present in the project area. ............................................................................................................................ 5 Step 2 - Review the Project for its Potential to Incidentally Take the Species ....................... 8 Step 3 - Review Measures to Avoid Incidental Take of the Rusty Patched Bumble Bee ...... 14 Conservation Measures ............................................................................................................ 15 Restore and Maintain High Quality Habitat -

Bumble Bees of CT-Females
Guide to CT Bumble Bees: Females This guide is only for females (12 antennal segments, 6 abdominal segments, most bumble bees, most have pollen baskets, no beards on their mandibles). First determine which yellow Three small eyes highlighted section your bee is in, then go through numbered characters to find a match. See if your bee matches the color patterns shown and the description in the text. Color patterns can vary. More detailed keys are available at discoverlife.org. Top of head Join the search for bumble bees with www.bumbleebeewatch.org Front of face Cheek Yellow hairs between wings, 1st abdominal band yellow (may have black spot in center of thorax) 1. Black on sides of 2nd ab, yellow or rusty in center 2.All other ab segments black 3. 2nd ab brownish centrally surrounded by yellow 2nd abdominal 2nd abdominal Light lemon Center spot band with yellow band with yellow hairs on on thorax with in middle, black yellow in middle top of head and sometimes faint V on sides. Yellow bordered by and on thorax. shaped extension often in a “W” rusty brown in a back from the shape. Top of swooping shape. middle. Queens head yellow. Top of head do not have black. Bombus impatiens Bombus affinis brownish central rusty patched bumble bee Bombus bimaculatus Bombus griseocollis common eastern bumble bee patch. two-spotted bumble bee brown-belted bumble bee 4. 2nd ab entirely yellow and ab 3-6 black 5. No obvious spot on thorax. Yellow on top Black on top of Variable color of head. -

TPM/IPM Weekly Report for Arborists, Landscape Managers & Nursery Managers
TPM/IPM Weekly Report for Arborists, Landscape Managers & Nursery Managers Commercial Horticulture July 12, 2019 In This Issue... Coordinator Weekly IPM Report: Stanton Gill, Extension Specialist, IPM for Nursery, Greenhouse and Managed - Cannibalistic predators Landscapes, [email protected]. 301-596-9413 (office) or 410-868-9400 (cell) - Impatiens downy mildew - Peach tree borer - Perennial program Regular Contributors: - Seed pods on magnolia Pest and Beneficial Insect Information: Stanton Gill and Paula Shrewsbury (Extension - Summer fruit tree pruning Specialists) and Nancy Harding, Faculty Research Assistant - Chiggers Disease Information: Karen Rane (Plant Pathologist) and David Clement (Extension - Powdery mildew on crape Specialist) myrtle Weed of the Week: Chuck Schuster (Extension Educator, Montgomery County) - Crape myrtle problems Cultural Information: Ginny Rosenkranz (Extension Educator, Wicomico/Worcester/ - Orangestriped oakworm Somerset Counties) - Japanese beetles and green Fertility Management: Andrew Ristvey (Extension Specialist, Wye Research & June beetles Education Center) - Redheaded pine sawflies Design, Layout and Editing: Suzanne Klick (Technician, CMREC) - Fall webworms - Yellownecked caterpillars - Rust and powdery mildew Cannibalistic Predators - Barklice (pscocids) By: Stanton Gill - Photos: deer, fall webworm adult, dobsonfly, tobacco Marie Rojas, IPM Scout, sent in hornworm, and mites some pictures of lady bird beetle Beneficial of the Week: Black larvae feeding on eggs in the cluster and gold bumble bee from which they hatched. I put these Weed of the Week: Small pictures out to entomologists across carpetgrass the US and received responses Plant of the Week: Prunus laurocerasus ‘schipkaensis’ noting that this activity is common. Pest Predictions Robin Rosetta sent in a picture from Degree Days Oregon with a larvae feeding on its Announcements sibling’s egg. -

An Abstract of the Thesis Of
AN ABSTRACT OF THE THESIS OF Sarah A. Maxfield-Taylor for the degree of Master of Science in Entomology presented on March 26, 2014. Title: Natural Enemies of Native Bumble Bees (Hymenoptera: Apidae) in Western Oregon Abstract approved: _____________________________________________ Sujaya U. Rao Bumble bees (Hymenoptera: Apidae) are important native pollinators in wild and agricultural systems, and are one of the few groups of native bees commercially bred for use in the pollination of a range of crops. In recent years, declines in bumble bees have been reported globally. One factor implicated in these declines, believed to affect bumble bee colonies in the wild and during rearing, is natural enemies. A diversity of fungi, protozoa, nematodes, and parasitoids has been reported to affect bumble bees, to varying extents, in different parts of the world. In contrast to reports of decline elsewhere, bumble bees have been thriving in Oregon on the West Coast of the U.S.A.. In particular, the agriculturally rich Willamette Valley in the western part of the state appears to be fostering several species. Little is known, however, about the natural enemies of bumble bees in this region. The objectives of this thesis were to: (1) identify pathogens and parasites in (a) bumble bees from the wild, and (b) bumble bees reared in captivity and (2) examine the effects of disease on bee hosts. Bumble bee queens and workers were collected from diverse locations in the Willamette Valley, in spring and summer. Bombus mixtus, Bombus nevadensis, and Bombus vosnesenskii collected from the wild were dissected and examined for pathogens and parasites, and these organisms were identified using morphological and molecular characteristics. -

Bumble Bee Surveys in the Columbia River Gorge National Scenic Area of Oregon and Washington
Bumble Bee Surveys in the Columbia River Gorge National Scenic Area of Oregon and Washington Final report from the Xerces Society to the U.S. Forest Service and Interagency Special Status/Sensitive Species Program (ISSSSP) Agreement L13AC00102, Modification 5 Bombus vosnesenskii on Balsamorhiza sagittata. Photo by Rich Hatfield, the Xerces Society. By Rich Hatfield, Sarina Jepsen, and Scott Black, the Xerces Society for Invertebrate Conservation September 2017 1 Table of Contents Abstract ......................................................................................................................................................... 3 Introduction .................................................................................................................................................. 3 Methods ........................................................................................................................................................ 6 Site Selection ............................................................................................................................................. 6 Site Descriptions (west to east) ................................................................................................................ 7 T14ES27 (USFS) ..................................................................................................................................... 7 Cape Horn (USFS) ................................................................................................................................. -

Bumble Bees Are Essential
Prepared by the Bombus Task Force of the North American Pollinator Protection Campaign (NAPPC) Photo Sheila Colla Rusty-patched bumble bee, Bombus affi nis Declining North American Bumble Bees Photo David Inouye Western bumble bee, Bombus occidentalis Bumble Bees Helping Photo James Strange are Pollinators Essential Thrive Franklin bumble bee, Bombus franklini Bumble Bee Facts Photo Leif Richardson Globally, there are about 250 described species Some bumble bee are known to rob fl owers of bumble bees. They are found primarily in the of their nectar. Nectar robbing occurs when temperate zones of North and South America, a bee extracts nectar from a fl ower without and Eurasia. coming into contact with its reproductive Yellow-banded bumble bee, Bumble bees are documented to pollinate parts (i.e. anthers and/or stigma), usually Bombus terricola many important food crops. They are also more by biting a hole at the base of the fl ower. Photo Ron Hemberger effective than honey bees at pollinating crops Bumble bees are effective buzz pollinators grown in greenhouses. of several economically important plants in When most insects are inactive due to cold the family Solanaceae such as tomato, bell temperatures bumble bees are able to fl y by pepper and eggplant. In buzz pollination warming their fl ight muscles by shivering, bees extract pollen from a fl ower by American bumble bee, enabling them to raise their body temperature vibrating against the fl ower’s anthers, Bombus pensylvanicus as necessary for fl ight. making an audible buzzing noise. Instead of starting their own colonies, some Currently, the Common Eastern bumble bee bumble bee species have evolved to take over (Bombus impatiens) is the only species being another species’ colony to rear their young.