Diet of Juvenile and Subadult White Sturgeon in The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Suisun Marsh Fish Report 2015 Final
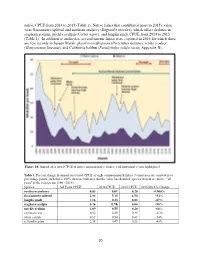
native CPUE from 2014 to 2015 (Table 1). Native fishes that contributed most to 2015's value were Sacramento splittail and northern anchovy (Engraulis mordax), which offset declines in staghorn sculpin, prickly sculpin (Cottus asper), and longfin smelt CPUE from 2014 to 2015 (Table 1). In addition to anchovies, several marine fishes were captured in 2016 for which there are few records in Suisun Marsh: plainfin midshipman (Porichthys notatus), white croaker (Genyonemus lineatus), and California halibut (Paralichthys californicus; Appendix B). Figure 14. Annual otter trawl CPUE of native and non-native fishes, with important events highlighted. Table 1. Percent change in annual otter trawl CPUE of eight common marsh fishes (% increases are equivalent to percentage points, such that a 100% increase indicates that the value has doubled; species in bold are native; "all years" is the average for 1980 - 2015). Species All Years CPUE 2014 CPUE 2015 CPUE 2015/2014 % Change northern anchovy 0.03 0.01 0.20 +1900% Sacramento splittail 2.84 5.15 6.90 +34% longfin smelt 1.16 0.23 0.03 -87% staghorn sculpin 0.26 0.16 0.00 -98% prickly sculpin 1.09 0.55 0.20 -64% common carp 0.52 0.49 0.19 -61% white catfish 0.63 0.94 0.43 -54% yellowfin goby 2.31 0.47 0.26 -45% 20 Beach Seines Annual beach seine CPUE in 2015 was similar to the average from 1980 to 2015 (57 fish per seine; Figure 15), declining mildly from 2014 to 2015 (62 and 52 per seine, respectively). CPUE declined slightly for both non-native and native fishes from 2014 to 2015 (Figure 15); as usual, non-native fish, dominated by Mississippi silversides (Menidia audens), were far more abundant in seine hauls than native fish (Table 2). -

Crangon Franciscorum Class: Multicrustacea, Malacostraca, Eumalacostraca
Phylum: Arthropoda, Crustacea Crangon franciscorum Class: Multicrustacea, Malacostraca, Eumalacostraca Order: Eucarida, Decapoda, Pleocyemata, Caridea Common gray shrimp Family: Crangonoidea, Crangonidae Taxonomy: Schmitt (1921) described many duncle segment (Wicksten 2011). Inner fla- shrimp in the genus Crago (e.g. Crago fran- gellum of the first antenna is greater than ciscorum) and reserved the genus Crangon twice as long as the outer flagellum (Kuris et for the snapping shrimp (now in the genus al. 2007) (Fig. 2). Alpheus). In 1955–56, the International Mouthparts: The mouth of decapod Commission on Zoological Nomenclature crustaceans comprises six pairs of appendag- formally reserved the genus Crangon for the es including one pair of mandibles (on either sand shrimps only. Recent taxonomic de- side of the mouth), two pairs of maxillae and bate revolves around potential subgeneric three pairs of maxillipeds. The maxillae and designation for C. franciscorum (C. Neocran- maxillipeds attach posterior to the mouth and gon franciscorum, C. franciscorum francis- extend to cover the mandibles (Ruppert et al. corum) (Christoffersen 1988; Kuris and Carl- 2004). Third maxilliped setose and with exo- ton 1977; Butler 1980; Wicksten 2011). pod in C. franciscorum and C. alaskensis (Wicksten 2011). Description Carapace: Thin and smooth, with a Size: Average body length is 49 mm for single medial spine (compare to Lissocrangon males and 68 mm for females (Wicksten with no gastric spines). Also lateral (Schmitt 2011). 1921) (Fig. 1), hepatic, branchiostegal and Color: White, mottled with small black spots, pterygostomian spines (Wicksten 2011). giving gray appearance. Rostrum: Rostrum straight and up- General Morphology: The body of decapod turned (Crangon, Kuris and Carlton 1977). -

OREGON ESTUARINE INVERTEBRATES an Illustrated Guide to the Common and Important Invertebrate Animals
OREGON ESTUARINE INVERTEBRATES An Illustrated Guide to the Common and Important Invertebrate Animals By Paul Rudy, Jr. Lynn Hay Rudy Oregon Institute of Marine Biology University of Oregon Charleston, Oregon 97420 Contract No. 79-111 Project Officer Jay F. Watson U.S. Fish and Wildlife Service 500 N.E. Multnomah Street Portland, Oregon 97232 Performed for National Coastal Ecosystems Team Office of Biological Services Fish and Wildlife Service U.S. Department of Interior Washington, D.C. 20240 Table of Contents Introduction CNIDARIA Hydrozoa Aequorea aequorea ................................................................ 6 Obelia longissima .................................................................. 8 Polyorchis penicillatus 10 Tubularia crocea ................................................................. 12 Anthozoa Anthopleura artemisia ................................. 14 Anthopleura elegantissima .................................................. 16 Haliplanella luciae .................................................................. 18 Nematostella vectensis ......................................................... 20 Metridium senile .................................................................... 22 NEMERTEA Amphiporus imparispinosus ................................................ 24 Carinoma mutabilis ................................................................ 26 Cerebratulus californiensis .................................................. 28 Lineus ruber ......................................................................... -

Bering Sea Marine Invasive Species Assessment Alaska Center for Conservation Science
Bering Sea Marine Invasive Species Assessment Alaska Center for Conservation Science Scientific Name: Palaemon macrodactylus Phylum Arthropoda Common Name oriental shrimp Class Malacostraca Order Decapoda Family Palaemonidae Z:\GAP\NPRB Marine Invasives\NPRB_DB\SppMaps\PALMAC.pn g 40 Final Rank 49.87 Data Deficiency: 3.75 Category Scores and Data Deficiencies Total Data Deficient Category Score Possible Points Distribution and Habitat: 20 26 3.75 Anthropogenic Influence: 6.75 10 0 Biological Characteristics: 20.5 30 0 Impacts: 0.75 30 0 Figure 1. Occurrence records for non-native species, and their geographic proximity to the Bering Sea. Ecoregions are based on the classification system by Spalding et al. (2007). Totals: 48.00 96.25 3.75 Occurrence record data source(s): NEMESIS and NAS databases. General Biological Information Tolerances and Thresholds Minimum Temperature (°C) 2 Minimum Salinity (ppt) 0.7 Maximum Temperature (°C) 33 Maximum Salinity (ppt) 51 Minimum Reproductive Temperature (°C) NA Minimum Reproductive Salinity (ppt) 3 Maximum Reproductive Temperature (°C) NA Maximum Reproductive Salinity (ppt) 34 Additional Notes Palaemon macrodactylus is commonly known as the Oriental shrimp. Its body is transparent with a reddish hue in the tail fan and antennary area. Females tend to be larger than males and have more pigmentation, with reddish spots all over their body, and a whitish longitudinal stripe that runs along the back. Females reach a maximum size of 45-70 mm, compared to 31.5-45 mm for males (Vazquez et al. 2012, qtd. in Fofnoff et al. 2003). Report updated on Wednesday, December 06, 2017 Page 1 of 13 1. -

Download Case
FOR PUBLICATION UNITED STATES COURT OF APPEALS FOR THE NINTH CIRCUIT SAN LUIS & DELTA-MENDOTA WATER AUTHORITY; WESTLANDS WATER DISTRICT, Plaintiffs-Appellants, and PIXLEY IRRIGATION DISTRICT; LOWER TULE RIVER IRRIGATION DISTRICT; TRI-VALLEY WATER dISTRICT; HILLS VALLEY IRRIGATION DISTRICT; KERN TULARE WATER DISTRICT; RAG GULCH WATER DISTRICT; STOCKTON EAST WATER DISTRICT; FRESNO COUNTY; TULARE COUNTY, Plaintiffs-Intervenors, and BAY INSTITUTE OF SAN FRANCISCO; SAVE SAN FRANCISCO BAY ASSOCIATION; ENVIRONMENTAL DEFENSE FUND; NATURAL RESOURCES DEFENSE COUNCIL; PACIFIC COAST FEDERATION OF FISHERMEN’S ASSOCIATIONS; INSTITUTE FOR FISHERIES RESOURCES; UNITED ANGLERS oF CALIFORNIA, Plaintiffs-Appellees, v. 2269 2270 SAN LUIS v. U.S. DEPARTMENT OF THE INTERIOR UNITED STATES OF AMERICA, DEPARTMENT OF THE INTERIOR, BUREAU OF RECLAMATION; KEN SALAZAR, SECRETARY OF THE INTERIOR; ROBYN THORSON, No. 09-17594 REGIONAL DIRECTOR OF THE UNITED D.C. No. STATES DEPARTMENT OF THE CV 97-06140- INTERIOR FISH AND WILDLIFE OWW SERVICE, REGION 1; DONALD GLASER, REGIONAL DIRECTOR, OPINION UNITED STATES DEPARTMENT OF THE INTERIOR BUREAU OF RECLAMATION, MID-PACIFIC REGION, Defendants-Appellees. Appeal from the United States District Court for the Eastern District of California Oliver W. Wanger, United States District Judge, Presiding Argued and Submitted March 15, 2011—Davis, California Filed March 2, 2012 Before: William A. Fletcher and Milan D. Smith, Jr., Circuit Judges, and George H. Wu, District Judge.* Opinion by Judge Wu; Partial Concurrence and Partial Dissent by Judge M. Smith *The Honorable George H. Wu, United States District Judge for the Central District of California, sitting by designation. 2274 SAN LUIS v. U.S. DEPARTMENT OF THE INTERIOR COUNSEL Thomas W. -

Acute Toxicity of Seven Alicyclic Hexanes to Striped Bass, Moron€ Saxatilis, and Bay Shrimp, Crangon Franciscorum, in Seawater
132 CALIFORNIA FISH AND GAME Cali(. FIsh and Game 71 (3): 132-140 1985 ACUTE TOXICITY OF SEVEN ALICYCLIC HEXANES TO STRIPED BASS, MORON€ SAXATILIS, AND BAY SHRIMP, CRANGON FRANCISCORUM, IN SEAWATER PETE E. BENVILLE, JR., JEANNETTE A. WHIPPLE, AND MAXWELL B. ELDRIDGE National Marine Fisheries Service Southwest Fisheries Center Tiburon Laboratory 31 50 Paradise Drive Tiburon, California 94920 Field monitoring studies have shown that many striped bass, Morone saxatilk, in California's Sacramento-San Joaquin Estuary are burdened with a wide variety of pollutants including many types of petroleum hydrocarbons. Alicyclic hydrocarbons are among these pollutants but there was no information on their toxicity to striped bass. Our studies were concerned with the acute toxicities of the simplest alicyclics in comparison to their counterparts in the aromatic series. The alicyclic compounds were cyclohexane, methylcyclohexane, ethylcyclohexane and four dimethylcy- clohexanes (1,l; 1,2; 19; and 1,4). Acute toxicities after 24 and 96 h exposures to seven alicyclic hexanes were determined for striped bass and one of their major food organisms, the bay shrimp, Crangon franciscorurn. The 96 h LCdfor striped bass and bay shrimp ranged from 3.2 to 9.3pl/l and from 1.0 to 6.2 pM, respectively. Slight differences were noted between the 24 and 96 h LCb values in all but two bioassays. Solubilities of these alicyclics in seawater and freshwater were determined since information in the literature was limited. Solubility was inversely related to the complexity of the alicyclic structure and ranged from 5.3 to 62 pl/l in distilled water and from 4.6 to 44 pl/l in seawater. -

An Overview of the Decapoda with Glossary and References
January 2011 Christina Ball Royal BC Museum An Overview of the Decapoda With Glossary and References The arthropods (meaning jointed leg) are a phylum that includes, among others, the insects, spiders, horseshoe crabs and crustaceans. A few of the traits that arthropods are characterized by are; their jointed legs, a hard exoskeleton made of chitin and growth by the process of ecdysis (molting). The Crustacea are a group nested within the Arthropoda which includes the shrimp, crabs, krill, barnacles, beach hoppers and many others. The members of this group present a wide range of morphology and life history, but they do have some unifying characteristics. They are the only group of arthropods that have two pairs of antenna. The decapods (meaning ten-legged) are a group within the Crustacea and are the topic of this key. The decapods are primarily characterized by a well developed carapace and ten pereopods (walking legs). The higher-level taxonomic groups within the Decapoda are the Dendrobranchiata, Anomura, Brachyura, Caridea, Astacidea, Axiidea, Gebiidea, Palinura and Stenopodidea. However, two of these groups, the Palinura (spiny lobsters) and the Stenopodidea (coral shrimps), do not occur in British Columbia and are not dealt with in this key. The remaining groups covered by this key include the crabs, hermit crabs, shrimp, prawns, lobsters, crayfish, mud shrimp, ghost shrimp and others. Arthropoda Crustacea Decapoda Dendrobranchiata – Prawns Caridea – Shrimp Astacidea – True lobsters and crayfish Thalassinidea - This group has recently -

1 Checklist of the Shrimps, Crabs, Lobsters and Crayfish of British Columbia 2011 (Order Decapoda) by Aaron Baldwin, Phd Candida
Checklist of the Shrimps, Crabs, Lobsters and Crayfish of British Columbia 2011 (Order Decapoda) by Aaron Baldwin, PhD Candidate School of Fisheries and Ocean Science University of Alaska, Fairbanks [email protected] The following list includes all decapod species known to have been found in British Columbia. The taxonomic scheme is the most currently accepted and follows the higher decapod classification of De Grave et al. (2009). Additional sources used in this classification include Bowman and Abele (1982), Abele and Felgenhauer (1986), Martin and Davis (2001), and Schram (2001). It is likely that further research will reveal additional species, both as range extensions and undescribed species. List revised April 30, 2011. Notable changes from earlier versions: The Superfamily Galatheoidea has been divided following the molecular taxonomies as suggested by Ahyong et al. (2009). This change has been verified by more recent work by Ahyong et al. (2010) and Schnabel et al. (2011). These works separate the Superfamily Chirostyloidea from the traditional galatheioids. Additionally these works change the higher taxonomies of the galatheioid families. Potential future taxonomic changes: Ahyong et al. (2009) in their molecular analysis of the infraorder Anomura found the superfamilies Paguroidea and Galatheoidea to be polyphyletic. The changes to the Paguroidea are not yet reflected in the taxonomic nomenclature, but are expected. Wicksten (2009) adopted the classification scheme of Christoffersen (1988) for the caridean family Hippolytidae -

BC Caridea Shrimp Key to Families Karl P. Kuchnow and Aaron
BC Caridea Shrimp Key to Families Karl P. Kuchnow and Aaron Baldwin Acknowledgements Special recognition must be given to the fine work of T.H. Butler (1980) on the shrimps of the Pacific Coast of Canada. The materials in that publication played a large role in the preparation of this on-line key. Family Acanthephyridae Acanthephyra chacei Krygier & Forss, 1981 Acanthephyra curtirostris Wood-Mason & Alcock, 1891 Peaked Shrimp Hymenodora acanthitelsonis Wasmer, 1972 Ambereye Shrimp Hymenodora frontalis Rathbun, 1902 Pacific Ambereye Hymenodora glacialis (Buchholz, 1874) Northern Ambereye Hymenodora gracilis Smith, 1886 Gracile Ambereye Notostomus japonicus Spence Bate, 1888 Japanese Spinyridge Family Alpheidae Betaeus harrimani Rathbun, 1904 Northern Hooded Shrimp Betaeus setosus J.F.L Hart, 1964 Fuzzy Hooded Shrimp Family Crangonidae Argis alaskensis (Kingsley, 1882) Alaskan Argid Argis crassa (Rathbun, 1899) Rough Argid Argis dentata (Rathbun, 1902) Arctic Argid Argis lar (Owen, 1839) Kuro Shrimp Argis levior (Rathbun, 1902) Nelson’s Argid Argis ovifer (Rathbun, 1902) Spliteye Argid Crangon alaskensis Lockington, 1877 Alaskan Bay Shrimp Crangon alba Holmes, 1900 Stout Crangon Crangon dalli Rathbun, 1902 Ridged Crangon Crangon franciscorum angustimana Rathbun, 1902 California Bay Shrimp Crangon franciscorum franciscorum Stimpson, 1856 California Bay Shrimp Crangon handi Curtis and Carlton, 1977 Bay Shrimp Crangon nigricauda Stimpson, 1856 Black Tailed Bay Shrimp Lissocrangon stylirostris (Holmes, 1900) Smooth Bay Shrimp Mesocrangon intermedia -

Crangon Franciscorum Class: Crustacea Sub-Class: Malacostraca Common Gray Shrimp Order: Decapoda, Natantia Family: Crangonidae
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by University of Oregon Scholars' Bank Phylum: Arthropoda Crangon franciscorum Class: Crustacea Sub-Class: Malacostraca Common gray shrimp Order: Decapoda, Natantia Family: Crangonidae Description Size—type: about 7.6 cm; South Slough (of Butler calls this species Crangon Coos Bay) specimen, female: 6.5 cm (Schmitt franciscorum franciscorum, to distinguish it 1921). from C.f. angustimana Rathbun 1902, the Color—white, mottled with small black spots, long-clawed Crangon (Butler 1980). This giving gray appearance; eyes salmon latter species lives in deeper water, and within (Schmitt 1921). a narrower range of temperatures than does Rostrum—short, flattened, rounded (fig. 2): C. f. franciscorum (Butler 1980). unornamented. Eyes—free, not covered by carapace: Ecological Information Crangon and Lissocrangon (Carlton and Kuris Range—southeastern Alaska to San Diego, 1975). California; type locality, San Francisco 3 Antennal Scale—about /4 the length of the (Schmitt 1921). carapace: blade broad, rounded and shorter Local Distribution—Yaquina Bay; South than spine (fig. 2). Slough (Collver Point, channel) (Krygier and Chelipeds—hands subchelate: Crangon and Horton 1975). Lissocrangon; hand (propodus) at least 4 Habitat—"sandy coves"; in bay channel, times as long as wide; finger closed nearly substrate of mud, rock (South Slough); also longitudinally (fig. 3) (Schmitt 1921). offshore (Schmitt 1921). Carapace—with a single medial spine: Salinity—collected at 30 ‰; determines Crangon and Lissocrangon; a pair of lateral distribution, (Krygier and Horton 1975). spines as well (Kuris and Carlton 1977). Temperature—great toleration of Abdomen—shrimp-like, with typical Caridean temperature variation; prefers warmer water bend; 2nd segment overlaps 1st (fig. -

Macrozooplankton and Micronekton of the Lower San Francisco Estuary: Seasonal, Interannual, and Regional Variation in Relation to Environmental Conditions
Estuaries Vol. 28, No. 3, p. 473-485 June 2005 Macrozooplankton and Micronekton of the Lower San Francisco Estuary: Seasonal, Interannual, and Regional Variation in Relation to Environmental Conditions DARREN S. GEWANT and STEPHEN M. BOLLENS* Romberg Tiburon Center for Environmental Studies and Department of Biology, San Francisco State University, 3152 Paradise Drive, Tiburon, California 94920 ABSTRACT: Macrozooplankton and micronekton are intermediaries linking lower trophic levels (e.g., phytoplankton and mesozooplankton) to higher ones (e.g., fishes and birds). These organisms have not been extensively studied in the San Francisco Estuary (SFE), California. The objective of this study was to determine the distribution and abundance of macrozooplankton and micronekton in the SFE and to describe how these vary seasonally, interannually, and regionally in relation to environmental variables. Water column macrozooplankton and micronekton were sampled monthly from September 1997 to December 2000 at 6 stations spanning North, Central, and South Bays using a Methot Trawl. The macrozooplankton and micronekton in the lower SFE were dominated by 4 fishes and 7 invertebrates that comprised 98% of the total catch. Correspondence analyses revealed 4 groups of species that exhibited similar patterns of distri- bution and abundance. The assemblages changed between the wet and dry seasons and with distance from the coastal ocean. Based on abundance patterns, the dominant taxa in the lower SFE can be classified as: organisms spawned from common members -

'Bay Shrimp' As a Term Refers to a Multiple Species of Crangon, Inclu
Fishery-at-a-Glance: Bay Shrimp Scientific Name(s): ‘Bay Shrimp’ as a term refers to a multiple species of Crangon, including the California Bay Shrimp, Crangon franciscorum, and other genera of shrimp targeted by trawlers in San Francisco Bay. Fish species also landed by this fishery are the Oriental Goby, Acanthogobius flavimanus, Longjaw Mudsucker, Gilichthys mirabilis, Staghorn Sculpin, Leptocottus armatus, and Plainfin Midshipman, Porichthys notatus. Range: The commercial Bay Shrimp fishery is confined to San Francisco Bay. Species fished under commercial Bay Shrimp regulations are also found in bays and estuaries from Alaska to San Diego. Habitat: Bay Shrimp are found in soft bottom estuaries. Size (length and weight): Average length for adult males and females are 45 and 60 millimeters (1.77 and 2.36 inch) respectively. Life span: Life spans of Bay Shrimp vary by estuary and in the San Francisco estuary, male shrimp are estimated to attain a maximum age of 1.5 year and females may live up to 2.5 years. Reproduction: The California Bay Shrimp, Blacktail Shrimp, and the Blackspotted Shrimp all display sexual dimorphism displaying distinguishable features after reaching a size of 22 mm (0.87 in) or more where the females carry their eggs externally. Prey: Bay Shrimp opportunistically prey upon small epibenthic and benthic fauna where they transfer energy from the primary consumers to apex predators in the food web. Predators: The various species of Bay Shrimp serve as a primary food source for commercial sport fishes in the estuaries of the Pacific Coast. Major predators include Green and White Sturgeon, Striped Bass, Leopard Shark, Brown Smoothhound Shark, Big Skate, White Croaker, Staghorn Sculpin, Starry Flounder, English Sole, Pile and Rubberlip Surfperch, Pacific Tomcod and Brown Rockfish.