Final Report on Intertidal Invertebrates in Tillamook Bay
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Appendix to Taxonomic Revision of Leopold and Rudolf Blaschkas' Glass Models of Invertebrates 1888 Catalogue, with Correction
http://www.natsca.org Journal of Natural Science Collections Title: Appendix to Taxonomic revision of Leopold and Rudolf Blaschkas’ Glass Models of Invertebrates 1888 Catalogue, with correction of authorities Author(s): Callaghan, E., Egger, B., Doyle, H., & E. G. Reynaud Source: Callaghan, E., Egger, B., Doyle, H., & E. G. Reynaud. (2020). Appendix to Taxonomic revision of Leopold and Rudolf Blaschkas’ Glass Models of Invertebrates 1888 Catalogue, with correction of authorities. Journal of Natural Science Collections, Volume 7, . URL: http://www.natsca.org/article/2587 NatSCA supports open access publication as part of its mission is to promote and support natural science collections. NatSCA uses the Creative Commons Attribution License (CCAL) http://creativecommons.org/licenses/by/2.5/ for all works we publish. Under CCAL authors retain ownership of the copyright for their article, but authors allow anyone to download, reuse, reprint, modify, distribute, and/or copy articles in NatSCA publications, so long as the original authors and source are cited. TABLE 3 – Callaghan et al. WARD AUTHORITY TAXONOMY ORIGINAL SPECIES NAME REVISED SPECIES NAME REVISED AUTHORITY N° (Ward Catalogue 1888) Coelenterata Anthozoa Alcyonaria 1 Alcyonium digitatum Linnaeus, 1758 2 Alcyonium palmatum Pallas, 1766 3 Alcyonium stellatum Milne-Edwards [?] Sarcophyton stellatum Kükenthal, 1910 4 Anthelia glauca Savigny Lamarck, 1816 5 Corallium rubrum Lamarck Linnaeus, 1758 6 Gorgonia verrucosa Pallas, 1766 [?] Eunicella verrucosa 7 Kophobelemon (Umbellularia) stelliferum -

Alexander the Great's Tombolos at Tyre and Alexandria, Eastern Mediterranean ⁎ N
Available online at www.sciencedirect.com Geomorphology 100 (2008) 377–400 www.elsevier.com/locate/geomorph Alexander the Great's tombolos at Tyre and Alexandria, eastern Mediterranean ⁎ N. Marriner a, , J.P. Goiran b, C. Morhange a a CNRS CEREGE UMR 6635, Université Aix-Marseille, Europôle de l'Arbois, BP 80, 13545 Aix-en-Provence cedex 04, France b CNRS MOM Archéorient UMR 5133, 5/7 rue Raulin, 69365 Lyon cedex 07, France Received 25 July 2007; received in revised form 10 January 2008; accepted 11 January 2008 Available online 2 February 2008 Abstract Tyre and Alexandria's coastlines are today characterised by wave-dominated tombolos, peculiar sand isthmuses that link former islands to the adjacent continent. Paradoxically, despite a long history of inquiry into spit and barrier formation, understanding of the dynamics and sedimentary history of tombolos over the Holocene timescale is poor. At Tyre and Alexandria we demonstrate that these rare coastal features are the heritage of a long history of natural morphodynamic forcing and human impacts. In 332 BC, following a protracted seven-month siege of the city, Alexander the Great's engineers cleverly exploited a shallow sublittoral sand bank to seize the island fortress; Tyre's causeway served as a prototype for Alexandria's Heptastadium built a few months later. We report stratigraphic and geomorphological data from the two sand spits, proposing a chronostratigraphic model of tombolo evolution. © 2008 Elsevier B.V. All rights reserved. Keywords: Tombolo; Spit; Tyre; Alexandria; Mediterranean; Holocene 1. Introduction Courtaud, 2000; Browder and McNinch, 2006); (2) establishing a typology of shoreline salients and tombolos (Zenkovich, 1967; The term tombolo is used to define a spit of sand or shingle Sanderson and Eliot, 1996); and (3) modelling the geometrical linking an island to the adjacent coast. -

A Radical Solution: the Phylogeny of the Nudibranch Family Fionidae
RESEARCH ARTICLE A Radical Solution: The Phylogeny of the Nudibranch Family Fionidae Kristen Cella1, Leila Carmona2*, Irina Ekimova3,4, Anton Chichvarkhin3,5, Dimitry Schepetov6, Terrence M. Gosliner1 1 Department of Invertebrate Zoology, California Academy of Sciences, San Francisco, California, United States of America, 2 Department of Marine Sciences, University of Gothenburg, Gothenburg, Sweden, 3 Far Eastern Federal University, Vladivostok, Russia, 4 Biological Faculty, Moscow State University, Moscow, Russia, 5 A.V. Zhirmunsky Instutute of Marine Biology, Russian Academy of Sciences, Vladivostok, Russia, 6 National Research University Higher School of Economics, Moscow, Russia a11111 * [email protected] Abstract Tergipedidae represents a diverse and successful group of aeolid nudibranchs, with approx- imately 200 species distributed throughout most marine ecosystems and spanning all bio- OPEN ACCESS geographical regions of the oceans. However, the systematics of this family remains poorly Citation: Cella K, Carmona L, Ekimova I, understood since no modern phylogenetic study has been undertaken to support any of the Chichvarkhin A, Schepetov D, Gosliner TM (2016) A Radical Solution: The Phylogeny of the proposed classifications. The present study is the first molecular phylogeny of Tergipedidae Nudibranch Family Fionidae. PLoS ONE 11(12): based on partial sequences of two mitochondrial (COI and 16S) genes and one nuclear e0167800. doi:10.1371/journal.pone.0167800 gene (H3). Maximum likelihood, maximum parsimony and Bayesian analysis were con- Editor: Geerat J. Vermeij, University of California, ducted in order to elucidate the systematics of this family. Our results do not recover the tra- UNITED STATES ditional Tergipedidae as monophyletic, since it belongs to a larger clade that includes the Received: July 7, 2016 families Eubranchidae, Fionidae and Calmidae. -

Marine Invertebrate Field Guide
Marine Invertebrate Field Guide Contents ANEMONES ....................................................................................................................................................................................... 2 AGGREGATING ANEMONE (ANTHOPLEURA ELEGANTISSIMA) ............................................................................................................................... 2 BROODING ANEMONE (EPIACTIS PROLIFERA) ................................................................................................................................................... 2 CHRISTMAS ANEMONE (URTICINA CRASSICORNIS) ............................................................................................................................................ 3 PLUMOSE ANEMONE (METRIDIUM SENILE) ..................................................................................................................................................... 3 BARNACLES ....................................................................................................................................................................................... 4 ACORN BARNACLE (BALANUS GLANDULA) ....................................................................................................................................................... 4 HAYSTACK BARNACLE (SEMIBALANUS CARIOSUS) .............................................................................................................................................. 4 CHITONS ........................................................................................................................................................................................... -

I © Copyright 2015 Kevin R. Turner
© Copyright 2015 Kevin R. Turner i Effects of fish predation on benthic communities in the San Juan Archipelago Kevin R. Turner A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy University of Washington 2015 Reading Committee: Kenneth P. Sebens, Chair Megan N. Dethier Daniel E. Schindler Program Authorized to Offer Degree: Biology ii University of Washington Abstract Effects of fish predation on benthic communities in the San Juan Archipelago Kevin R. Turner Chair of the Supervisory Committee: Professor Kenneth P. Sebens Department of Biology Predation is a strong driver of community assembly, particularly in marine systems. Rockfish and other large fishes are the dominant predators in the rocky subtidal habitats of the San Juan Archipelago in NW Washington State. Here I examine the consumptive effects of these predatory fishes, beginning with a study of rockfish diet, and following with tests of the direct influence of predation on prey species and the indirect influence on other community members. In the first chapter I conducted a study of the diet of copper rockfish. Food web models benefit from recent and local data, and in this study I compared my findings with historic diet data from the Salish Sea and other localities along the US West Coast. Additionally, non-lethal methods of diet sampling are necessary to protect depleted rockfish populations, and I successfully used gastric lavage to sample these fish. Copper rockfish from this study fed primarily on shrimp and other demersal crustaceans, and teleosts made up a very small portion of their diet. Compared to previous studies, I found much higher consumption of shrimp and much iii lower consumption of teleosts, a difference that is likely due in part to geographic or temporal differences in prey availability. -

Balanus Glandula Class: Multicrustacea, Hexanauplia, Thecostraca, Cirripedia
Phylum: Arthropoda, Crustacea Balanus glandula Class: Multicrustacea, Hexanauplia, Thecostraca, Cirripedia Order: Thoracica, Sessilia, Balanomorpha Acorn barnacle Family: Balanoidea, Balanidae, Balaninae Description (the plate overlapping plate edges) and radii Size: Up to 3 cm in diameter, but usually (the plate edge marked off from the parietes less than 1.5 cm (Ricketts and Calvin 1971; by a definite change in direction of growth Kozloff 1993). lines) (Fig. 3b) (Newman 2007). The plates Color: Shell usually white, often irregular themselves include the carina, the carinola- and color varies with state of erosion. Cirri teral plates and the compound rostrum (Fig. are black and white (see Plate 11, Kozloff 3). 1993). Opercular Valves: Valves consist of General Morphology: Members of the Cirri- two pairs of movable plates inside the wall, pedia, or barnacles, can be recognized by which close the aperture: the tergum and the their feathery thoracic limbs (called cirri) that scutum (Figs. 3a, 4, 5). are used for feeding. There are six pairs of Scuta: The scuta have pits on cirri in B. glandula (Fig. 1). Sessile barna- either side of a short adductor ridge (Fig. 5), cles are surrounded by a shell that is com- fine growth ridges, and a prominent articular posed of a flat basis attached to the sub- ridge. stratum, a wall formed by several articulated Terga: The terga are the upper, plates (six in Balanus species, Fig. 3) and smaller plate pair and each tergum has a movable opercular valves including terga short spur at its base (Fig. 4), deep crests for and scuta (Newman 2007) (Figs. -

Nudibranchia: Flabellinidae) from the Red and Arabian Seas
Ruthenica, 2020, vol. 30, No. 4: 183-194. © Ruthenica, 2020 Published online October 1, 2020. http: ruthenica.net Molecular data and updated morphological description of Flabellina rubrolineata (Nudibranchia: Flabellinidae) from the Red and Arabian seas Irina A. EKIMOVA1,5, Tatiana I. ANTOKHINA2, Dimitry M. SCHEPETOV1,3,4 1Lomonosov Moscow State University, Leninskie Gory 1-12, 119234 Moscow, RUSSIA; 2A.N. Severtsov Institute of Ecology and Evolution, Leninskiy prosp. 33, 119071 Moscow, RUSSIA; 3N.K. Koltzov Institute of Developmental Biology RAS, Vavilov str. 26, 119334 Moscow, RUSSIA; 4Moscow Power Engineering Institute (MPEI, National Research University), 111250 Krasnokazarmennaya 14, Moscow, RUSSIA. 5Corresponding author; E-mail: [email protected] ABSTRACT. Flabellina rubrolineata was believed to have a wide distribution range, being reported from the Mediterranean Sea (non-native), the Red Sea, the Indian Ocean and adjacent seas, and the Indo-West Pacific and from Australia to Hawaii. In the present paper, we provide a redescription of Flabellina rubrolineata, based on specimens collected near the type locality of this species in the Red Sea. The morphology of this species was studied using anatomical dissections and scanning electron microscopy. To place this species in the phylogenetic framework and test the identity of other specimens of F. rubrolineata from the Indo-West Pacific we sequenced COI, H3, 16S and 28S gene fragments and obtained phylogenetic trees based on Bayesian and Maximum likelihood inferences. Our morphological and molecular results show a clear separation of F. rubrolineata from the Red Sea from its relatives in the Indo-West Pacific. We suggest that F. rubrolineata is restricted to only the Red Sea, the Arabian Sea and the Mediterranean Sea and to West Indian Ocean, while specimens from other regions belong to a complex of pseudocryptic species. -
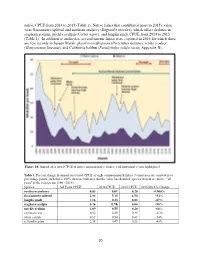
Suisun Marsh Fish Report 2015 Final
native CPUE from 2014 to 2015 (Table 1). Native fishes that contributed most to 2015's value were Sacramento splittail and northern anchovy (Engraulis mordax), which offset declines in staghorn sculpin, prickly sculpin (Cottus asper), and longfin smelt CPUE from 2014 to 2015 (Table 1). In addition to anchovies, several marine fishes were captured in 2016 for which there are few records in Suisun Marsh: plainfin midshipman (Porichthys notatus), white croaker (Genyonemus lineatus), and California halibut (Paralichthys californicus; Appendix B). Figure 14. Annual otter trawl CPUE of native and non-native fishes, with important events highlighted. Table 1. Percent change in annual otter trawl CPUE of eight common marsh fishes (% increases are equivalent to percentage points, such that a 100% increase indicates that the value has doubled; species in bold are native; "all years" is the average for 1980 - 2015). Species All Years CPUE 2014 CPUE 2015 CPUE 2015/2014 % Change northern anchovy 0.03 0.01 0.20 +1900% Sacramento splittail 2.84 5.15 6.90 +34% longfin smelt 1.16 0.23 0.03 -87% staghorn sculpin 0.26 0.16 0.00 -98% prickly sculpin 1.09 0.55 0.20 -64% common carp 0.52 0.49 0.19 -61% white catfish 0.63 0.94 0.43 -54% yellowfin goby 2.31 0.47 0.26 -45% 20 Beach Seines Annual beach seine CPUE in 2015 was similar to the average from 1980 to 2015 (57 fish per seine; Figure 15), declining mildly from 2014 to 2015 (62 and 52 per seine, respectively). CPUE declined slightly for both non-native and native fishes from 2014 to 2015 (Figure 15); as usual, non-native fish, dominated by Mississippi silversides (Menidia audens), were far more abundant in seine hauls than native fish (Table 2). -

Crangon Franciscorum Class: Multicrustacea, Malacostraca, Eumalacostraca
Phylum: Arthropoda, Crustacea Crangon franciscorum Class: Multicrustacea, Malacostraca, Eumalacostraca Order: Eucarida, Decapoda, Pleocyemata, Caridea Common gray shrimp Family: Crangonoidea, Crangonidae Taxonomy: Schmitt (1921) described many duncle segment (Wicksten 2011). Inner fla- shrimp in the genus Crago (e.g. Crago fran- gellum of the first antenna is greater than ciscorum) and reserved the genus Crangon twice as long as the outer flagellum (Kuris et for the snapping shrimp (now in the genus al. 2007) (Fig. 2). Alpheus). In 1955–56, the International Mouthparts: The mouth of decapod Commission on Zoological Nomenclature crustaceans comprises six pairs of appendag- formally reserved the genus Crangon for the es including one pair of mandibles (on either sand shrimps only. Recent taxonomic de- side of the mouth), two pairs of maxillae and bate revolves around potential subgeneric three pairs of maxillipeds. The maxillae and designation for C. franciscorum (C. Neocran- maxillipeds attach posterior to the mouth and gon franciscorum, C. franciscorum francis- extend to cover the mandibles (Ruppert et al. corum) (Christoffersen 1988; Kuris and Carl- 2004). Third maxilliped setose and with exo- ton 1977; Butler 1980; Wicksten 2011). pod in C. franciscorum and C. alaskensis (Wicksten 2011). Description Carapace: Thin and smooth, with a Size: Average body length is 49 mm for single medial spine (compare to Lissocrangon males and 68 mm for females (Wicksten with no gastric spines). Also lateral (Schmitt 2011). 1921) (Fig. 1), hepatic, branchiostegal and Color: White, mottled with small black spots, pterygostomian spines (Wicksten 2011). giving gray appearance. Rostrum: Rostrum straight and up- General Morphology: The body of decapod turned (Crangon, Kuris and Carlton 1977). -

Systematics, Phylogeny, and Taphonomy of Ghost Shrimps (Decapoda): a Perspective from the Fossil Record
73 (3): 401 – 437 23.12.2015 © Senckenberg Gesellschaft für Naturforschung, 2015. Systematics, phylogeny, and taphonomy of ghost shrimps (Decapoda): a perspective from the fossil record Matúš Hyžný *, 1, 2 & Adiël A. Klompmaker 3 1 Geological-Paleontological Department, Natural History Museum Vienna, Burgring 7, 1010 Vienna, Austria; Matúš Hyžný [hyzny.matus@ gmail.com] — 2 Department of Geology and Paleontology, Faculty of Natural Sciences, Comenius University, Mlynská dolina, Ilkovičova 6, SVK-842 15 Bratislava, Slovakia — 3 Florida Museum of Natural History, University of Florida, 1659 Museum Road, PO Box 117800, Gaines- ville, FL 32611, USA; Adiël A. Klompmaker [[email protected]] — * Correspond ing author Accepted 06.viii.2015. Published online at www.senckenberg.de/arthropod-systematics on 14.xii.2015. Editor in charge: Stefan Richter. Abstract Ghost shrimps of Callianassidae and Ctenochelidae are soft-bodied, usually heterochelous decapods representing major bioturbators of muddy and sandy (sub)marine substrates. Ghost shrimps have a robust fossil record spanning from the Early Cretaceous (~ 133 Ma) to the Holocene and their remains are present in most assemblages of Cenozoic decapod crustaceans. Their taxonomic interpretation is in flux, mainly because the generic assignment is hindered by their insufficient preservation and disagreement in the biological classification. Fur- thermore, numerous taxa are incorrectly classified within the catch-all taxonCallianassa . To show the historical patterns in describing fos- sil ghost shrimps and to evaluate taphonomic aspects influencing the attribution of ghost shrimp remains to higher level taxa, a database of all fossil species treated at some time as belonging to the group has been compiled: 250 / 274 species are considered valid ghost shrimp taxa herein. -

The Barnacle Amphibalanus Improvisus (Darwin, 1854), and the Mitten Crab Eriocheir: One Invasive Species Getting Off on Another!
BioInvasions Records (2015) Volume 4, Issue 3: 205–209 Open Access doi: http://dx.doi.org/10.3391/bir.2015.4.3.09 © 2015 The Author(s). Journal compilation © 2015 REABIC Rapid Communication The barnacle Amphibalanus improvisus (Darwin, 1854), and the mitten crab Eriocheir: one invasive species getting off on another! Murtada D. Naser1,4, Philip S. Rainbow2, Paul F. Clark2*, Amaal Gh. Yasser1,4 and Diana S. Jones3 1Marine Biology Department, Marine Science Centre, University of Basrah, Basrah, Iraq 2Department of Life Sciences, Natural History Museum, Cromwell Road, London SW7 5BD, England 3Western Australian Museum, 49 Kew Street, Welshpool, Western Australia, 6106 Australia 4Australian Rivers Institute, Griffith University, 170 Kessels Road, Nathan, Queensland, 4111 Australia E-mail: [email protected] (MDN), [email protected] (PSR), [email protected] (PFC), [email protected] (AGY), [email protected] (DSJ) *Corresponding author Received: 9 March 2015 / Accepted: 20 May 2015 / Published online: 16 June 2015 Handling editor: Vadim Panov Abstract The balanoid barnacle, Amphibalanus improvisus (Darwin, 1854), was found on the carapaces of two invasive species of mitten crabs: Eriocheir sinensis H. Milne Edwards, 1853 and E. hepuensis Dai, 1991. The first instance was from a female mitten crab captured from the River Thames estuary, Kent, England, where A. improvisus is common. However, the second record, on a Hepu mitten crab from Iraq is the first record of A. improvisus from the Persian Gulf. Key words: Eriocheir sinensis H. Milne Edwards, 1853, E. hepuensis Dai, 1991, invasive species, England, Iraq, barnacles, mitten crabs Introduction the eastern and western North Atlantic; Baltic Sea; west coast of Africa (to the Cape of Good “Hairy” (Southeast and East Asia) or “mitten” Hope); Mediterranean Sea; Black Sea; Caspian (Europe) crabs are currently assigned to Eriocheir Sea; Red Sea; Straits of Malacca; Singapore; De Haan, 1835 (Brachyura: Grapsoidea: Varunidae). -

Appendix 3 Marine Spcies Lists
Appendix 3 Marine Species Lists with Abundance and Habitat Notes for Provincial Helliwell Park Marine Species at “Wall” at Flora Islet and Reef Marine Species at Norris Rocks Marine Species at Toby Islet Reef Marine Species at Maude Reef, Lambert Channel Habitats and Notes of Marine Species of Helliwell Provincial Park Helliwell Provincial Park Ecosystem Based Plan – March 2001 Marine Species at wall at Flora Islet and Reef Common Name Latin Name Abundance Notes Sponges Cloud sponge Aphrocallistes vastus Abundant, only local site occurance Numerous, only local site where Chimney sponge, Boot sponge Rhabdocalyptus dawsoni numerous Numerous, only local site where Chimney sponge, Boot sponge Staurocalyptus dowlingi numerous Scallop sponges Myxilla, Mycale Orange ball sponge Tethya californiana Fairly numerous Aggregated vase sponge Polymastia pacifica One sighting Hydroids Sea Fir Abietinaria sp. Corals Orange sea pen Ptilosarcus gurneyi Numerous Orange cup coral Balanophyllia elegans Abundant Zoanthids Epizoanthus scotinus Numerous Anemones Short plumose anemone Metridium senile Fairly numerous Giant plumose anemone Metridium gigantium Fairly numerous Aggregate green anemone Anthopleura elegantissima Abundant Tube-dwelling anemone Pachycerianthus fimbriatus Abundant Fairly numerous, only local site other Crimson anemone Cribrinopsis fernaldi than Toby Islet Swimming anemone Stomphia sp. Fairly numerous Jellyfish Water jellyfish Aequoria victoria Moon jellyfish Aurelia aurita Lion's mane jellyfish Cyanea capillata Particuilarly abundant