Atomic and Nuclear Physics Atomic Structure
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

B2.IV Nuclear and Particle Physics
B2.IV Nuclear and Particle Physics A.J. Barr February 13, 2014 ii Contents 1 Introduction 1 2 Nuclear 3 2.1 Structure of matter and energy scales . 3 2.2 Binding Energy . 4 2.2.1 Semi-empirical mass formula . 4 2.3 Decays and reactions . 8 2.3.1 Alpha Decays . 10 2.3.2 Beta decays . 13 2.4 Nuclear Scattering . 18 2.4.1 Cross sections . 18 2.4.2 Resonances and the Breit-Wigner formula . 19 2.4.3 Nuclear scattering and form factors . 22 2.5 Key points . 24 Appendices 25 2.A Natural units . 25 2.B Tools . 26 2.B.1 Decays and the Fermi Golden Rule . 26 2.B.2 Density of states . 26 2.B.3 Fermi G.R. example . 27 2.B.4 Lifetimes and decays . 27 2.B.5 The flux factor . 28 2.B.6 Luminosity . 28 2.C Shell Model § ............................. 29 2.D Gamma decays § ............................ 29 3 Hadrons 33 3.1 Introduction . 33 3.1.1 Pions . 33 3.1.2 Baryon number conservation . 34 3.1.3 Delta baryons . 35 3.2 Linear Accelerators . 36 iii CONTENTS CONTENTS 3.3 Symmetries . 36 3.3.1 Baryons . 37 3.3.2 Mesons . 37 3.3.3 Quark flow diagrams . 38 3.3.4 Strangeness . 39 3.3.5 Pseudoscalar octet . 40 3.3.6 Baryon octet . 40 3.4 Colour . 41 3.5 Heavier quarks . 43 3.6 Charmonium . 45 3.7 Hadron decays . 47 Appendices 48 3.A Isospin § ................................ 49 3.B Discovery of the Omega § ...................... -
Pair Energy of Proton and Neutron in Atomic Nuclei
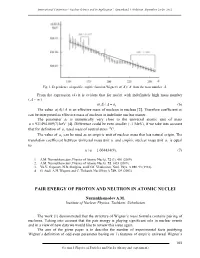
International Conference “Nuclear Science and its Application”, Samarkand, Uzbekistan, September 25-28, 2012 Fig. 1. Dependence of specific empiric function Wigner's a()/ A A from the mass number A. From the expression (4) it is evident that for nuclei with indefinitely high mass number ( A ~ ) a()/. A A a1 (6) The value a()/ A A is an effective mass of nucleon in nucleus [2]. Therefore coefficient a1 can be interpreted as effective mass of nucleon in indefinite nuclear matter. The parameter a1 is numerically very close to the universal atomic unit of mass u 931494.009(7) keV [4]. Difference could be even smaller (~1 MeV), if we take into account that for definition of u, used mass of neutral atom 12C. The value of a1 can be used as an empiric unit of nuclear mass that has natural origin. The translation coefficient between universal mass unit u and empiric nuclear mass unit a1 is equal to: u/ a1 1.004434(9). (7) 1. A.M. Nurmukhamedov, Physics of Atomic Nuclei, 72 (3), 401 (2009). 2. A.M. Nurmukhamedov, Physics of Atomic Nuclei, 72, 1435 (2009). 3. Yu.V. Gaponov, N.B. Shulgina, and D.M. Vladimirov, Nucl. Phys. A 391, 93 (1982). 4. G. Audi, A.H. Wapstra and C. Thibault, Nucl.Phys A 729, 129 (2003). PAIR ENERGY OF PROTON AND NEUTRON IN ATOMIC NUCLEI Nurmukhamedov A.M. Institute of Nuclear Physics, Tashkent, Uzbekistan The work [1] demonstrated that the structure of Wigner’s mass formula contains pairing of nucleons. Taking into account that the pair energy is playing significant role in nuclear events and in a view of new data we would like to review this issue again. -

Prospects for Measurements with Strange Hadrons at Lhcb
Prospects for measurements with strange hadrons at LHCb A. A. Alves Junior1, M. O. Bettler2, A. Brea Rodr´ıguez1, A. Casais Vidal1, V. Chobanova1, X. Cid Vidal1, A. Contu3, G. D'Ambrosio4, J. Dalseno1, F. Dettori5, V.V. Gligorov6, G. Graziani7, D. Guadagnoli8, T. Kitahara9;10, C. Lazzeroni11, M. Lucio Mart´ınez1, M. Moulson12, C. Mar´ınBenito13, J. Mart´ınCamalich14;15, D. Mart´ınezSantos1, J. Prisciandaro 1, A. Puig Navarro16, M. Ramos Pernas1, V. Renaudin13, A. Sergi11, K. A. Zarebski11 1Instituto Galego de F´ısica de Altas Enerx´ıas(IGFAE), Santiago de Compostela, Spain 2Cavendish Laboratory, University of Cambridge, Cambridge, United Kingdom 3INFN Sezione di Cagliari, Cagliari, Italy 4INFN Sezione di Napoli, Napoli, Italy 5Oliver Lodge Laboratory, University of Liverpool, Liverpool, United Kingdom, now at Universit`adegli Studi di Cagliari, Cagliari, Italy 6LPNHE, Sorbonne Universit´e,Universit´eParis Diderot, CNRS/IN2P3, Paris, France 7INFN Sezione di Firenze, Firenze, Italy 8Laboratoire d'Annecy-le-Vieux de Physique Th´eorique , Annecy Cedex, France 9Institute for Theoretical Particle Physics (TTP), Karlsruhe Institute of Technology, Kalsruhe, Germany 10Institute for Nuclear Physics (IKP), Karlsruhe Institute of Technology, Kalsruhe, Germany 11School of Physics and Astronomy, University of Birmingham, Birmingham, United Kingdom 12INFN Laboratori Nazionali di Frascati, Frascati, Italy 13Laboratoire de l'Accelerateur Lineaire (LAL), Orsay, France 14Instituto de Astrof´ısica de Canarias and Universidad de La Laguna, Departamento de Astrof´ısica, La Laguna, Tenerife, Spain 15CERN, CH-1211, Geneva 23, Switzerland 16Physik-Institut, Universit¨atZ¨urich,Z¨urich,Switzerland arXiv:1808.03477v2 [hep-ex] 31 Jul 2019 Abstract This report details the capabilities of LHCb and its upgrades towards the study of kaons and hyperons. -

Dark Matter in Nuclear Physics
Dark Matter in Nuclear Physics George Fuller (UCSD), Andrew Hime (LANL), Reyco Henning (UNC), Darin Kinion (LLNL), Spencer Klein (LBNL), Stefano Profumo (Caltech) Michael Ramsey-Musolf (Caltech/UW Madison), Robert Stokstad (LBNL) Introduction A transcendent accomplishment of nuclear physics and observational cosmology has been the definitive measurement of the baryon content of the universe. Big Bang Nucleosynthesis calculations, combined with measurements made with the largest new telescopes of the primordial deuterium abundance, have inferred the baryon density of the universe. This result has been confirmed by observations of the anisotropies in the Cosmic Microwave Background. The surprising upshot is that baryons account for only a small fraction of the observed mass and energy in the universe. It is now firmly established that most of the mass-energy in the Universe is comprised of non-luminous and unknown forms. One component of this is the so- called “dark energy”, believed to be responsible for the observed acceleration of the expansion rate of the universe. Another component appears to be composed of non-luminous material with non-relativistic kinematics at the current epoch. The relationship between the dark matter and the dark energy is unknown. Several national studies and task-force reports have found that the identification of the mysterious “dark matter” is one of the most important pursuits in modern science. It is now clear that an explanation for this phenomenon will require some sort of new physics, likely involving a new particle or particles, and in any case involving physics beyond the Standard Model. A large number of dark matter studies, from theory to direct dark matter particle detection, involve nuclear physics and nuclear physicists. -

Lepton Probes in Nuclear Physics
L C f' - l ■) aboratoire ATIONAI FR9601644 ATURNE 91191 Gif-sur-Yvette Cedex France LEPTON PROBES IN NUCLEAR PHYSICS J. ARVIEUX Laboratoire National Saturne IN2P3-CNRS A DSM-CEA, CESaclay F-9I191 Gif-sur-Yvette Cedex, France EPS Conference : Large Facilities in Physics, Lausanne (Switzerland) Sept. 12-14, 1994 ££)\-LNS/Ph/94-18 Centre National de la Recherche Scientifique CBD Commissariat a I’Energie Atomique VOL LEPTON PROBES IN NUCLEAR PHYSICS J. ARVTEUX Laboratoire National Saturne IN2P3-CNRS &. DSM-CEA, CESaclay F-91191 Gif-sur-Yvette Cedex, France ABSTRACT 1. Introduction This review concerns the facilities which use the lepton probe to learn about nuclear physics. Since this Conference is attended by a large audience coming from diverse horizons, a few definitions may help to explain what I am going to talk about. 1.1. Leptons versus hadrons The particle physics world is divided in leptons and hadrons. Leptons are truly fundamental particles which are point-like (their dimension cannot be measured) and which interact with matter through two well-known forces : the electromagnetic interaction and the weak interaction which have been regrouped in the 70's in the single electroweak interaction following the theoretical insight of S. Weinberg (Nobel prize in 1979) and the experimental discoveries of the Z° and W±- bosons at CERN by C. Rubbia and Collaborators (Nobel prize in 1984). The leptons comprise 3 families : electrons (e), muons (jt) and tau (r) and their corresponding neutrinos : ve, and vr . Nuclear physics can make use of electrons and muons but since muons are produced at large energy accelerators, they more or less belong to the particle world although they can also be used to study solid state physics. -

The Nuclear Physics of Neutron Stars
The Nuclear Physics of Neutron Stars Exotic Beam Summer School 2015 Florida State University Tallahassee, FL - August 2015 J. Piekarewicz (FSU) Neutron Stars EBSS 2015 1 / 21 My FSU Collaborators My Outside Collaborators Genaro Toledo-Sanchez B. Agrawal (Saha Inst.) Karim Hasnaoui M. Centelles (U. Barcelona) Bonnie Todd-Rutel G. Colò (U. Milano) Brad Futch C.J. Horowitz (Indiana U.) Jutri Taruna W. Nazarewicz (MSU) Farrukh Fattoyev N. Paar (U. Zagreb) Wei-Chia Chen M.A. Pérez-Garcia (U. Raditya Utama Salamanca) P.G.- Reinhard (U. Erlangen-Nürnberg) X. Roca-Maza (U. Milano) D. Vretenar (U. Zagreb) J. Piekarewicz (FSU) Neutron Stars EBSS 2015 2 / 21 S. Chandrasekhar and X-Ray Chandra White dwarfs resist gravitational collapse through electron degeneracy pressure rather than thermal pressure (Dirac and R.H. Fowler 1926) During his travel to graduate school at Cambridge under Fowler, Chandra works out the physics of the relativistic degenerate electron gas in white dwarf stars (at the age of 19!) For masses in excess of M =1:4 M electrons becomes relativistic and the degeneracy pressure is insufficient to balance the star’s gravitational attraction (P n5=3 n4=3) ∼ ! “For a star of small mass the white-dwarf stage is an initial step towards complete extinction. A star of large mass cannot pass into the white-dwarf stage and one is left speculating on other possibilities” (S. Chandrasekhar 1931) Arthur Eddington (1919 bending of light) publicly ridiculed Chandra’s on his discovery Awarded the Nobel Prize in Physics (in 1983 with W.A. Fowler) In 1999, NASA lunches “Chandra” the premier USA X-ray observatory J. -

Introduction to Nuclear Physics and Nuclear Decay
NM Basic Sci.Intro.Nucl.Phys. 06/09/2011 Introduction to Nuclear Physics and Nuclear Decay Larry MacDonald [email protected] Nuclear Medicine Basic Science Lectures September 6, 2011 Atoms Nucleus: ~10-14 m diameter ~1017 kg/m3 Electron clouds: ~10-10 m diameter (= size of atom) water molecule: ~10-10 m diameter ~103 kg/m3 Nucleons (protons and neutrons) are ~10,000 times smaller than the atom, and ~1800 times more massive than electrons. (electron size < 10-22 m (only an upper limit can be estimated)) Nuclear and atomic units of length 10-15 = femtometer (fm) 10-10 = angstrom (Å) Molecules mostly empty space ~ one trillionth of volume occupied by mass Water Hecht, Physics, 1994 (wikipedia) [email protected] 2 [email protected] 1 NM Basic Sci.Intro.Nucl.Phys. 06/09/2011 Mass and Energy Units and Mass-Energy Equivalence Mass atomic mass unit, u (or amu): mass of 12C ≡ 12.0000 u = 19.9265 x 10-27 kg Energy Electron volt, eV ≡ kinetic energy attained by an electron accelerated through 1.0 volt 1 eV ≡ (1.6 x10-19 Coulomb)*(1.0 volt) = 1.6 x10-19 J 2 E = mc c = 3 x 108 m/s speed of light -27 2 mass of proton, mp = 1.6724x10 kg = 1.007276 u = 938.3 MeV/c -27 2 mass of neutron, mn = 1.6747x10 kg = 1.008655 u = 939.6 MeV/c -31 2 mass of electron, me = 9.108x10 kg = 0.000548 u = 0.511 MeV/c [email protected] 3 Elements Named for their number of protons X = element symbol Z (atomic number) = number of protons in nucleus N = number of neutrons in nucleus A A A A (atomic mass number) = Z + N Z X N Z X X [A is different than, but approximately equal to the atomic weight of an atom in amu] Examples; oxygen, lead A Electrically neural atom, Z X N has Z electrons in its 16 208 atomic orbit. -

Module01 Nuclear Physics and Reactor Theory
Module I Nuclear physics and reactor theory International Atomic Energy Agency, May 2015 v1.0 Background In 1991, the General Conference (GC) in its resolution RES/552 requested the Director General to prepare 'a comprehensive proposal for education and training in both radiation protection and in nuclear safety' for consideration by the following GC in 1992. In 1992, the proposal was made by the Secretariat and after considering this proposal the General Conference requested the Director General to prepare a report on a possible programme of activities on education and training in radiological protection and nuclear safety in its resolution RES1584. In response to this request and as a first step, the Secretariat prepared a Standard Syllabus for the Post- graduate Educational Course in Radiation Protection. Subsequently, planning of specialised training courses and workshops in different areas of Standard Syllabus were also made. A similar approach was taken to develop basic professional training in nuclear safety. In January 1997, Programme Performance Assessment System (PPAS) recommended the preparation of a standard syllabus for nuclear safety based on Agency Safely Standard Series Documents and any other internationally accepted practices. A draft Standard Syllabus for Basic Professional Training Course in Nuclear Safety (BPTC) was prepared by a group of consultants in November 1997 and the syllabus was finalised in July 1998 in the second consultants meeting. The Basic Professional Training Course on Nuclear Safety was offered for the first time at the end of 1999, in English, in Saclay, France, in cooperation with Institut National des Sciences et Techniques Nucleaires/Commissariat a l'Energie Atomique (INSTN/CEA). -

S Ince the Brilliant Insights and Pioneering
The secret life of quarks ince the brilliant insights and pioneering experiments of Geiger, S Marsden and Rutherford first revealed that atoms contain nuclei [1], nuclear physics has developed into an astoundingly rich and diverse field. We have probed the complexities of protons and nuclei in our laboratories; we have seen how nuclear processes writ large in the cosmos are key to our existence; and we have found a myriad of ways to harness the nuclear realm for energy, medical and security applications. Nevertheless, many open questions still remain in nuclear physics— the mysterious and hidden world that is the domain of quarks and gluons. 44 ) detmold mit physics annual 2017 The secret life by William of quarks Detmold The modern picture of an atom includes multiple layers of structure: atoms are composed of nuclei and electrons; nuclei are composed of protons and neutrons; and protons and neutrons are themselves composed of quarks—matter particles—that are held together by force- carrying particles called gluons. And it is the primary goal of the Large Hadron Collider (LHC) in Geneva, Switzerland, to investigate the tantalizing possibility that an even deeper structure exists. Despite this understanding, we are only just beginning to be able to predict the properties and interactions of nuclei from the underlying physics of quarks and gluons, as these fundamental constituents are never seen directly in experiments but always remain confined inside composite particles (hadrons), such as the proton. Recent progress in supercomputing and algorithm development has changed this, and we are rapidly approaching the point where precision calculations of the simplest nuclear processes will be possible. -

B Meson Decays Marina Artuso1, Elisabetta Barberio2 and Sheldon Stone*1
Review Open Access B meson decays Marina Artuso1, Elisabetta Barberio2 and Sheldon Stone*1 Address: 1Department of Physics, Syracuse University, Syracuse, NY 13244, USA and 2School of Physics, University of Melbourne, Victoria 3010, Australia Email: Marina Artuso - [email protected]; Elisabetta Barberio - [email protected]; Sheldon Stone* - [email protected] * Corresponding author Published: 20 February 2009 Received: 20 February 2009 Accepted: 20 February 2009 PMC Physics A 2009, 3:3 doi:10.1186/1754-0410-3-3 This article is available from: http://www.physmathcentral.com/1754-0410/3/3 © 2009 Stone et al This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/ licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract We discuss the most important Physics thus far extracted from studies of B meson decays. Measurements of the four CP violating angles accessible in B decay are reviewed as well as direct CP violation. A detailed discussion of the measurements of the CKM elements Vcb and Vub from semileptonic decays is given, and the differences between resulting values using inclusive decays versus exclusive decays is discussed. Measurements of "rare" decays are also reviewed. We point out where CP violating and rare decays could lead to observations of physics beyond that of the Standard Model in future experiments. If such physics is found by directly observation of new particles, e.g. in LHC experiments, B decays can play a decisive role in interpreting the nature of these particles. -

Introduction to PHY008: Atomic and Nuclear Physics
PHY008 Prof. L. Roszkowski, F9c, (23580), [email protected] (EMAIL MAY BE BEST WAY TO CONTACT ME) Introduction to PHY008: Atomic and Nuclear Physics The greatest advances in technology have taken place in the last hundred years. In 1897 few would have imagined that the probing of materials at the atomic level would reveal so much. These early discoveries of atomic constituents and their structure would pave the way for semi-conductor electronics, develop key concepts in physical laws, and offer a replacement energy source for fossil fuels in the form of nuclear power. This course summarises key discoveries in early particle physics and combines historical background with the detailed physics understanding needed to fully appreciate the subject. While lectures may impart knowledge, the skills necessary to apply this knowledge to real cases can only be obtained by practising. Examples will be given in each lecture but a huge collection of further questions, some with full solutions, are provided for problem class sessions. You are advised to try as many of these problems as you can. If you can do these questions, then the exam will be easy!!! Recommended books The notes are pretty complete I hope, so there are no compulsory textbooks. Any A level textbook would be helpful and the following are recommended though not essential. Cutnell and Johnson, Physics, (Wiley, 6th edition or more recent). Adams and Allday, Advanced Physics (Advanced Science), (Oxford), 2000. Lectures This topic will be covered in12 lectures in Lecture Theatre A every Thursday during term-time from 12:10am until 13:00pm. -

Mass of the Neutron 1.00893, Table 1 Gives the Binding Energies of the Nuclei up to He4 on the Supposition That the Constituents Are Protons and Neutrons
VOL. 32, 1946 PHYSICS: E. E. WITMER 283 INTEGRAL AND RATIONAL NUMBERS IN THE NUCLEAR DOMAIN AND THEIR SIGNIFICANCE By ENOS E. WITMER RANDAL MORGAN LABORATORY OF PHYSICS, UNIVERSITY OF PENNSYLVANIA Communicated September 30, 1946 Using the value of the fine structure constant given by Birgel in 1941 hc/e2 = 2r/ax = 860.986 =1= 0.101. (1) This number is so very close to an integer that it is natural to assume that hc/e2 = 861 (2) exactly. In this paper we shall assume that equation (2) is-true and significant. Note that 861 = 1/2(42 X 41) (3) so that 861 is of the form 1/2n (n - 1) with n = 42. Both these numbers 861 and 42 turn up in very interesting ways in connection with nuclei and elementary particles, as will be shown in the sequel. Also 42 = 6 X 7 and the number 7 also appears frequently in connection with some of these quantities. The quantities concerned are the masses and binding energies of nuclei, both in the ground state and in excited states, the magnetic moments of nuclei and the ratios of the masses of the proton and neutron to that of the electron. The mass of the H' atom on the physical scale is 1.00813 = 0.000017 according to Birge.' Subtracting the mass of the electron on the physical scale, the mass of the proton is Mp= 1.00758 = 0.000017 m. u. (4) Now 2Mp/861 = 0.00234049 m. u. (5) which is the binding energy of the deuteron within the limits of experi- mental error.