Mass of the Neutron 1.00893, Table 1 Gives the Binding Energies of the Nuclei up to He4 on the Supposition That the Constituents Are Protons and Neutrons
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neutron Stars
Chandra X-Ray Observatory X-Ray Astronomy Field Guide Neutron Stars Ordinary matter, or the stuff we and everything around us is made of, consists largely of empty space. Even a rock is mostly empty space. This is because matter is made of atoms. An atom is a cloud of electrons orbiting around a nucleus composed of protons and neutrons. The nucleus contains more than 99.9 percent of the mass of an atom, yet it has a diameter of only 1/100,000 that of the electron cloud. The electrons themselves take up little space, but the pattern of their orbit defines the size of the atom, which is therefore 99.9999999999999% Chandra Image of Vela Pulsar open space! (NASA/PSU/G.Pavlov et al. What we perceive as painfully solid when we bump against a rock is really a hurly-burly of electrons moving through empty space so fast that we can't see—or feel—the emptiness. What would matter look like if it weren't empty, if we could crush the electron cloud down to the size of the nucleus? Suppose we could generate a force strong enough to crush all the emptiness out of a rock roughly the size of a football stadium. The rock would be squeezed down to the size of a grain of sand and would still weigh 4 million tons! Such extreme forces occur in nature when the central part of a massive star collapses to form a neutron star. The atoms are crushed completely, and the electrons are jammed inside the protons to form a star composed almost entirely of neutrons. -

The Five Common Particles
The Five Common Particles The world around you consists of only three particles: protons, neutrons, and electrons. Protons and neutrons form the nuclei of atoms, and electrons glue everything together and create chemicals and materials. Along with the photon and the neutrino, these particles are essentially the only ones that exist in our solar system, because all the other subatomic particles have half-lives of typically 10-9 second or less, and vanish almost the instant they are created by nuclear reactions in the Sun, etc. Particles interact via the four fundamental forces of nature. Some basic properties of these forces are summarized below. (Other aspects of the fundamental forces are also discussed in the Summary of Particle Physics document on this web site.) Force Range Common Particles It Affects Conserved Quantity gravity infinite neutron, proton, electron, neutrino, photon mass-energy electromagnetic infinite proton, electron, photon charge -14 strong nuclear force ≈ 10 m neutron, proton baryon number -15 weak nuclear force ≈ 10 m neutron, proton, electron, neutrino lepton number Every particle in nature has specific values of all four of the conserved quantities associated with each force. The values for the five common particles are: Particle Rest Mass1 Charge2 Baryon # Lepton # proton 938.3 MeV/c2 +1 e +1 0 neutron 939.6 MeV/c2 0 +1 0 electron 0.511 MeV/c2 -1 e 0 +1 neutrino ≈ 1 eV/c2 0 0 +1 photon 0 eV/c2 0 0 0 1) MeV = mega-electron-volt = 106 eV. It is customary in particle physics to measure the mass of a particle in terms of how much energy it would represent if it were converted via E = mc2. -

Photons from 252Cf and 241Am-Be Neutron Sources H
Neutronenbestrahlungsraum Calibration Laboratory LB6411 Am-Be Quelle [email protected] Photons from 252Cf and 241Am-Be neutron sources H. Hoedlmoser, M. Boschung, K. Meier and S. Mayer Paul Scherrer Institut, CH-5232 Villigen PSI, Switzerland At the accredited PSI calibration laboratory neutron reference fields traceable to the standards of the Physikalisch-Technische Bundesanstalt (PTB) in Germany are available for the calibration of ambient and personal dose equivalent (rate) meters and passive dosimeters. The photon contribution to H*(10) in the neutron fields of the 252Cf and 241Am-Be sources was measured using various photon dose rate meters and active and passive dosimeters. Measuring photons from a neutron source involves considerable uncertainties due to the presence of neutrons, due to a non-zero neutron sensitivity of the photon detector and due to the energy response of the photon detectors. Therefore eight independent detectors and methods were used to obtain a reliable estimate for the photon contribution as an average of the individual methods. For the 241Am-Be source a photon contribution of approximately 4.9% was determined and for the 252Cf source a contribution of 3.6%. 1) Photon detectors 2) Photons from 252Cf and 241Am-Be neutron sources Figure 1 252Cf decays through -emission (~97%) and through spontaneous fission (~3%) with a half-life of 2.65 y. Both processes are accompanied by photon radiation. Furthermore the spectrum of fission products contains radioactive elements that produce additional gamma photons. In the 241Am-Be source 241Am decays through -emission and various low energy emissions: 241Am237Np + + with a half life of 432.6 y. -

B2.IV Nuclear and Particle Physics
B2.IV Nuclear and Particle Physics A.J. Barr February 13, 2014 ii Contents 1 Introduction 1 2 Nuclear 3 2.1 Structure of matter and energy scales . 3 2.2 Binding Energy . 4 2.2.1 Semi-empirical mass formula . 4 2.3 Decays and reactions . 8 2.3.1 Alpha Decays . 10 2.3.2 Beta decays . 13 2.4 Nuclear Scattering . 18 2.4.1 Cross sections . 18 2.4.2 Resonances and the Breit-Wigner formula . 19 2.4.3 Nuclear scattering and form factors . 22 2.5 Key points . 24 Appendices 25 2.A Natural units . 25 2.B Tools . 26 2.B.1 Decays and the Fermi Golden Rule . 26 2.B.2 Density of states . 26 2.B.3 Fermi G.R. example . 27 2.B.4 Lifetimes and decays . 27 2.B.5 The flux factor . 28 2.B.6 Luminosity . 28 2.C Shell Model § ............................. 29 2.D Gamma decays § ............................ 29 3 Hadrons 33 3.1 Introduction . 33 3.1.1 Pions . 33 3.1.2 Baryon number conservation . 34 3.1.3 Delta baryons . 35 3.2 Linear Accelerators . 36 iii CONTENTS CONTENTS 3.3 Symmetries . 36 3.3.1 Baryons . 37 3.3.2 Mesons . 37 3.3.3 Quark flow diagrams . 38 3.3.4 Strangeness . 39 3.3.5 Pseudoscalar octet . 40 3.3.6 Baryon octet . 40 3.4 Colour . 41 3.5 Heavier quarks . 43 3.6 Charmonium . 45 3.7 Hadron decays . 47 Appendices 48 3.A Isospin § ................................ 49 3.B Discovery of the Omega § ...................... -
Pair Energy of Proton and Neutron in Atomic Nuclei
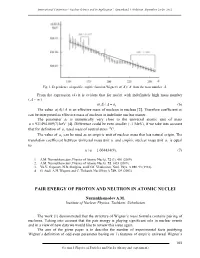
International Conference “Nuclear Science and its Application”, Samarkand, Uzbekistan, September 25-28, 2012 Fig. 1. Dependence of specific empiric function Wigner's a()/ A A from the mass number A. From the expression (4) it is evident that for nuclei with indefinitely high mass number ( A ~ ) a()/. A A a1 (6) The value a()/ A A is an effective mass of nucleon in nucleus [2]. Therefore coefficient a1 can be interpreted as effective mass of nucleon in indefinite nuclear matter. The parameter a1 is numerically very close to the universal atomic unit of mass u 931494.009(7) keV [4]. Difference could be even smaller (~1 MeV), if we take into account that for definition of u, used mass of neutral atom 12C. The value of a1 can be used as an empiric unit of nuclear mass that has natural origin. The translation coefficient between universal mass unit u and empiric nuclear mass unit a1 is equal to: u/ a1 1.004434(9). (7) 1. A.M. Nurmukhamedov, Physics of Atomic Nuclei, 72 (3), 401 (2009). 2. A.M. Nurmukhamedov, Physics of Atomic Nuclei, 72, 1435 (2009). 3. Yu.V. Gaponov, N.B. Shulgina, and D.M. Vladimirov, Nucl. Phys. A 391, 93 (1982). 4. G. Audi, A.H. Wapstra and C. Thibault, Nucl.Phys A 729, 129 (2003). PAIR ENERGY OF PROTON AND NEUTRON IN ATOMIC NUCLEI Nurmukhamedov A.M. Institute of Nuclear Physics, Tashkent, Uzbekistan The work [1] demonstrated that the structure of Wigner’s mass formula contains pairing of nucleons. Taking into account that the pair energy is playing significant role in nuclear events and in a view of new data we would like to review this issue again. -

Opportunities for Neutrino Physics at the Spallation Neutron Source (SNS)
Opportunities for Neutrino Physics at the Spallation Neutron Source (SNS) Paper submitted for the 2008 Carolina International Symposium on Neutrino Physics Yu Efremenko1,2 and W R Hix2,1 1University of Tennessee, Knoxville TN 37919, USA 2Oak Ridge National Laboratory, Oak Ridge TN 37981, USA E-mail: [email protected] Abstract. In this paper we discuss opportunities for a neutrino program at the Spallation Neutrons Source (SNS) being commissioning at ORNL. Possible investigations can include study of neutrino-nuclear cross sections in the energy rage important for supernova dynamics and neutrino nucleosynthesis, search for neutrino-nucleus coherent scattering, and various tests of the standard model of electro-weak interactions. 1. Introduction It seems that only yesterday we gathered together here at Columbia for the first Carolina Neutrino Symposium on Neutrino Physics. To my great astonishment I realized it was already eight years ago. However by looking back we can see that enormous progress has been achieved in the field of neutrino science since that first meeting. Eight years ago we did not know which region of mixing parameters (SMA. LMA, LOW, Vac) [1] would explain the solar neutrino deficit. We did not know whether this deficit is due to neutrino oscillations or some other even more exotic phenomena, like neutrinos decay [2], or due to the some other effects [3]. Hints of neutrino oscillation of atmospheric neutrinos had not been confirmed in accelerator experiments. Double beta decay collaborations were just starting to think about experiments with sensitive masses of hundreds of kilograms. Eight years ago, very few considered that neutrinos can be used as a tool to study the Earth interior [4] or for non- proliferation [5]. -
Proton Therapy ACKNOWLEDGEMENTS
AMERICAN BRAIN TUMOR ASSOCIATION Proton Therapy ACKNOWLEDGEMENTS ABOUT THE AMERICAN BRAIN TUMOR ASSOCIATION Founded in 1973, the American Brain Tumor Association (ABTA) was the first national nonprofit organization dedicated solely to brain tumor research. For over 40 years, the Chicago-based ABTA has been providing comprehensive resources that support the complex needs of brain tumor patients and caregivers, as well as the critical funding of research in the pursuit of breakthroughs in brain tumor diagnosis, treatment and care. To learn more about the ABTA, visit www.abta.org. We gratefully acknowledge Anita Mahajan, Director of International Development, MD Anderson Proton Therapy Center, director, Pediatric Radiation Oncology, co-section head of Pediatric and CNS Radiation Oncology, The University of Texas MD Anderson Cancer Center; Kevin S. Oh, MD, Department of Radiation Oncology, Massachusetts General Hospital; and Sridhar Nimmagadda, PhD, assistant professor of Radiology, Medicine and Oncology, Johns Hopkins University, for their review of this edition of this publication. This publication is not intended as a substitute for professional medical advice and does not provide advice on treatments or conditions for individual patients. All health and treatment decisions must be made in consultation with your physician(s), utilizing your specific medical information. Inclusion in this publication is not a recommendation of any product, treatment, physician or hospital. COPYRIGHT © 2015 ABTA REPRODUCTION WITHOUT PRIOR WRITTEN PERMISSION IS PROHIBITED AMERICAN BRAIN TUMOR ASSOCIATION Proton Therapy INTRODUCTION Brain tumors are highly variable in their treatment and prognosis. Many are benign and treated conservatively, while others are malignant and require aggressive combinations of surgery, radiation and chemotherapy. -
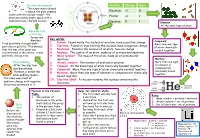
Key Words: 1. Proton: Found Inside the Nucleus of an Atom, Have a Positive Charge 2. Electron: Found in Rings Orbiting the Nucle
Nucleus development Particle Charge Mass This experiment allowed Rutherford to replace the plum pudding Electron -1 0 model with the nuclear model – the Proton +1 1 atom was mainly empty space with a small positively charged nucleus Neutron 0 1 Element All the same type of atom Alpha particle scattering Geiger and Key words: Marsden 1. Proton: Found inside the nucleus of an atom, have a positive charge Compound fired positively-charged alpha More than one type 2. Electron: Found in rings orbiting the nucleus, have a negative charge particles at gold foil. This showed of atom chemically that the mas of an atom was 3. Neutrons: Found in the nucleus of an atom, have no charge bonded together concentrated in the centre, it was 4. Nucleus: The centre of an atom, made up of protons and neutrons positively charged too 5. Mass number: The mass of the atom, made up of protons and neutrons Mixture Plum pudding 6. Atomic number: The number of protons in an atom More than one type After the electron 7. Element: All the same type of atom chemically bonded together of element or was discovered, 8. Compound: More than one type of atom chemically bonded together compound not chemically bound Thomson created the 9. Mixture: More than one type of element or compound not chemically together plum pudding model – bound together the atom was a ball of 10. Electron Shell: A ring surrounding the nucleus containing the positive charge with negative electrons electrons scattered in it Position in the Periodic Rules for electron shells: Table: 1. -

Detection of a Strange Particle
10 extraordinary papers Within days, Watson and Crick had built a identify the full set of codons was completed in forensics, and research into more-futuristic new model of DNA from metal parts. Wilkins by 1966, with Har Gobind Khorana contributing applications, such as DNA-based computing, immediately accepted that it was correct. It the sequences of bases in several codons from is well advanced. was agreed between the two groups that they his experiments with synthetic polynucleotides Paradoxically, Watson and Crick’s iconic would publish three papers simultaneously in (see go.nature.com/2hebk3k). structure has also made it possible to recog- Nature, with the King’s researchers comment- With Fred Sanger and colleagues’ publica- nize the shortcomings of the central dogma, ing on the fit of Watson and Crick’s structure tion16 of an efficient method for sequencing with the discovery of small RNAs that can reg- to the experimental data, and Franklin and DNA in 1977, the way was open for the com- ulate gene expression, and of environmental Gosling publishing Photograph 51 for the plete reading of the genetic information in factors that induce heritable epigenetic first time7,8. any species. The task was completed for the change. No doubt, the concept of the double The Cambridge pair acknowledged in their human genome by 2003, another milestone helix will continue to underpin discoveries in paper that they knew of “the general nature in the history of DNA. biology for decades to come. of the unpublished experimental results and Watson devoted most of the rest of his ideas” of the King’s workers, but it wasn’t until career to education and scientific administra- Georgina Ferry is a science writer based in The Double Helix, Watson’s explosive account tion as head of the Cold Spring Harbor Labo- Oxford, UK. -

Prospects for Measurements with Strange Hadrons at Lhcb
Prospects for measurements with strange hadrons at LHCb A. A. Alves Junior1, M. O. Bettler2, A. Brea Rodr´ıguez1, A. Casais Vidal1, V. Chobanova1, X. Cid Vidal1, A. Contu3, G. D'Ambrosio4, J. Dalseno1, F. Dettori5, V.V. Gligorov6, G. Graziani7, D. Guadagnoli8, T. Kitahara9;10, C. Lazzeroni11, M. Lucio Mart´ınez1, M. Moulson12, C. Mar´ınBenito13, J. Mart´ınCamalich14;15, D. Mart´ınezSantos1, J. Prisciandaro 1, A. Puig Navarro16, M. Ramos Pernas1, V. Renaudin13, A. Sergi11, K. A. Zarebski11 1Instituto Galego de F´ısica de Altas Enerx´ıas(IGFAE), Santiago de Compostela, Spain 2Cavendish Laboratory, University of Cambridge, Cambridge, United Kingdom 3INFN Sezione di Cagliari, Cagliari, Italy 4INFN Sezione di Napoli, Napoli, Italy 5Oliver Lodge Laboratory, University of Liverpool, Liverpool, United Kingdom, now at Universit`adegli Studi di Cagliari, Cagliari, Italy 6LPNHE, Sorbonne Universit´e,Universit´eParis Diderot, CNRS/IN2P3, Paris, France 7INFN Sezione di Firenze, Firenze, Italy 8Laboratoire d'Annecy-le-Vieux de Physique Th´eorique , Annecy Cedex, France 9Institute for Theoretical Particle Physics (TTP), Karlsruhe Institute of Technology, Kalsruhe, Germany 10Institute for Nuclear Physics (IKP), Karlsruhe Institute of Technology, Kalsruhe, Germany 11School of Physics and Astronomy, University of Birmingham, Birmingham, United Kingdom 12INFN Laboratori Nazionali di Frascati, Frascati, Italy 13Laboratoire de l'Accelerateur Lineaire (LAL), Orsay, France 14Instituto de Astrof´ısica de Canarias and Universidad de La Laguna, Departamento de Astrof´ısica, La Laguna, Tenerife, Spain 15CERN, CH-1211, Geneva 23, Switzerland 16Physik-Institut, Universit¨atZ¨urich,Z¨urich,Switzerland arXiv:1808.03477v2 [hep-ex] 31 Jul 2019 Abstract This report details the capabilities of LHCb and its upgrades towards the study of kaons and hyperons. -

Improved Algorithms and Coupled Neutron-Photon Transport For
Submitted to ‘Chinese Physics C’ Improved Algorithms and Coupled Neutron-Photon Transport for Auto-Importance Sampling Method * Xin Wang(王鑫)1,2 Zhen Wu(武祯)3 Rui Qiu(邱睿)1,2;1) Chun-Yan Li(李春艳)3 Man-Chun Liang(梁漫春)1 Hui Zhang(张辉) 1,2 Jun-Li Li(李君利)1,2 Zhi Gang(刚直)4 Hong Xu(徐红)4 1 Department of Engineering Physics, Tsinghua University, Beijing 100084, China 2Key Laboratory of Particle & Radiation Imaging, Ministry of Education, Beijing 100084, China 3 Nuctech Company Limited, Beijing 100084, China 4 State Nuclear Hua Qing (Beijing) Nuclear Power Technology R&D Center Co. Ltd., Beijing 102209, China Abstract: The Auto-Importance Sampling (AIS) method is a Monte Carlo variance reduction technique proposed for deep penetration problems, which can significantly improve computational efficiency without pre-calculations for importance distribution. However, the AIS method is only validated with several simple examples, and cannot be used for coupled neutron-photon transport. This paper presents improved algorithms for the AIS method, including particle transport, fictitious particle creation and adjustment, fictitious surface geometry, random number allocation and calculation of the estimated relative error. These improvements allow the AIS method to be applied to complicated deep penetration problems with complex geometry and multiple materials. A Completely coupled Neutron-Photon Auto-Importance Sampling (CNP-AIS) method is proposed to solve the deep penetration problems of coupled neutron-photon transport using the improved algorithms. The NUREG/CR-6115 PWR benchmark was calculated by using the methods of CNP-AIS, geometry splitting with Russian roulette and analog Monte Carlo, respectively. -

8.1 the Neutron-To-Proton Ratio
M. Pettini: Introduction to Cosmology | Lecture 8 PRIMORDIAL NUCLEOSYNTHESIS Our discussion at the end of the previous lecture concentrated on the rela- tivistic components of the Universe, photons and leptons. Baryons (which are non-relativistic) did not figure because they make a trifling contribu- tion to the energy density. However, from t ∼ 1 s a number of nuclear reactions involving baryons took place. The end result of these reactions was to lock up most of the free neutrons into 4He nuclei and to create trace amounts of D, 3He, 7Li and 7Be. This is Primordial Nucleosynthesis, also referred to as Big Bang Nucleosynthesis (BBN). 8.1 The Neutron-to-Proton Ratio Before neutrino decoupling at around 1 MeV, neutrons and protons are kept in mutual thermal equilibrium through charged-current weak interactions: + n + e ! p +ν ¯e − p + e ! n + νe (8.1) − n ! p + e +ν ¯e While equilibrium persists, the relative number densities of neutrons and protons are given by a Boltzmann factor based on their mass difference: n ∆m c2 1:5 = exp − = exp − (8.2) p eq kT T10 where 10 ∆m = (mn − mp) = 1:29 MeV = 1:5 × 10 K (8.3) 10 and T10 is the temperature in units of 10 K. As discussed in Lecture 7.1.2, this equilibrium will be maintained so long as the timescale for the weak interactions is short compared with the timescale of the cosmic expansion (which increases the mean distance between parti- cles). In 7.2.1 we saw that the ratio of the two rates varies approximately as: Γ T 3 ' : (8.4) H 1:6 × 1010 K 1 This steep dependence on temperature can be appreciated as follows.