PPCO Twist System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Coordination Des Syndicats CGT
Coordination des syndicats CGT STRATEGIE DE LA DIRECTION DU GROUPE SANOFI CONSEQUENCES INDUSTRIELLES ET SOCIALES Document d’août 2014 1. Situation économique – Coût du capital p2 2. Evolution des effectifs – Bilan des restructurations majeures p3 3. Stratégie Sanofi 2009-2015 : Désengagement scientifique et industriel en Europe et plus particulièrement en France p4 4. Stratégie de structuration du groupe en entités qui peuvent être cédées, vendues, fermées, échangées. p6 5. Crédit d’impôt – Des aides publiques pour quel usage ? p7 6. Industrie pharmaceutique : des besoins fondamentaux p7 7. Interpellation des élus et du gouvernement p8 1. Situation économique – Coût du capital Première entreprise pharmaceutique française et européenne. Sanofi est issu de la fusion de nombreux laboratoires pharmaceutiques français dont les principaux étaient Roussel Uclaf, Rhône Poulenc, Synthelabo, Sanofi et de l’allemand Hoechst. Sanofi représente 30 à 40% du potentiel national (effectifs, sites, R&D,…) de l’industrie pharmaceutique française dans notre pays. L’avenir du groupe et de ses activités en France conditionne l’avenir de l’industrie pharmaceutique française et constitue un élément incontournable de l’indépendance thérapeutique du pays. Le C.A. de sanofi dans le monde sur 2013 a atteint 33 milliards € et devrait se situer à un niveau légèrement supérieur en 2014. Plusieurs médicaments de référence étant aujourd’hui tombés dans le domaine public, le chiffre d’affaires repart à la hausse. Le résultat net des activités a été de 6,8 milliards € en 2013 et les projections sur 2014 laissent envisager une progression de 5% de celui-ci. La rentabilité est estimée par les économistes parmi les meilleures de l’industrie pharmaceutique dans le monde. -

National Lipid Association Annual Summary of Clinical Lipidology 2016 Disclosures Dr. Harold E. Bays: Dr. Bays Discloses That He
National Lipid Association Annual Summary of Clinical Lipidology 2016 Disclosures Dr. Harold E. Bays: Dr. Bays discloses that he has received research grants from Arena Pharmaceuticals, Boehringer Ingelheim, Cargill Inc., GlaxoSmithKline, Novo Nordisk, Orexigen Therapeutics, Shionogi, Takeda, Stratum Nutrition, California Raisin Board, Esperion, Essentialis, Forest, Gilead Sciences Inc., Given, Hoffman-LaRoche, Home Access, Novartis, Omthera, Pfizer, Trygg Pharmaceuticals, TWI Bio, Xoma, Ardea Inc., High Point Pharmaceuticals LLC, Micropharma Limited, TransTech Pharma Inc., TIMI, Pozen, Regeneron, and Elcelyx. He further discloses that he has received honoraria/research grants from Amarin, Amgen, AstraZeneca, Bristol-Myers Squibb, Catabasis, Daiichi-Sankyo Inc., Eisai, Merck & Co, VIVUS, Zeomedex, and WPU. Dr. Peter H. Jones: Dr. Jones has received consulting or speaker honoraria from Merck and Co., Amgen, and Sanofi-Aventis/Regeneron. Dr. W. Virgil Brown: Dr. Brown is the editor of the Journal of Clinical Lipidology and further discloses that he has received consulting fees/honoraria from Akcea, Esperion, Regeneron, Amgen, Genzyme, Pfizer Inc., Merck and Co, GlaxoSmithKline, Medtelligence, and Vindico. Dr. Terry A. Jacobson: Dr. Jacobson has received consulting fees from Merck and Co., Amarin, Amgen, AstraZeneca, and Regeneron/Sanofi-Aventis. Dr. Karen E. Aspry: Dr. Aspry has no disclosures to report. Dr. Christie M. Ballantyne: Dr. Ballantyne has received research grants from Abbott Diagnostics, Amarin, Amgen, Eli Lilly, Esperion, ISIS Pharmaceuticals, Novartis Pharmaceuticals, Pfizer, Otsuka, Regeneron, Roche Diagnostic, Sanofi-Synthelabo, and Takeda Pharmaceuticals. He has also received consulting fees from Abbott Diagnostics, Amarin, Amgen, AstraZeneca, Eli Lilly, Esperion, Genzyme, ISIS Pharmaceuticals, Matinas BioPharma Inc., Merck and Co., Novartis Pharmaceuticals, Pfizer, Regeneron, Roche, and Sanofi-Synthelabo. -

Ce Que Sanofi Dit De La Politique Industrielle Française
Ce que Sanofi dit de la politique industrielle française mediapart.fr/journal/economie/030221/ce-que-sanofi-dit-de-la-politique-industrielle-francaise Martine Orange, Mediapart, 3 février 2021 Les salariés de Sanofi ont beau essayer de chercher des explications, ils ne comprennent pas. Ou plutôt ils ne comprennent que trop bien la conduite du groupe pharmaceutique. Après le revers de sa stratégie dans l’élaboration d’un vaccin contre le Covid-19, repoussé désormais au mieux à la fin de l’année, tout aurait dû pousser la direction de Sanofi à s’interroger sur la pertinence de ses choix, sur la place laissée à la recherche jugée comme essentielle. Mais rien ne s’est passé. Le 28 janvier, la direction de Sanofi Recherche et Développement en France a confirmé à l’occasion d’un comité social d’entreprise (CSE) la suppression de 364 emplois en France, une mesure qui vise particulièrement l’unité de Strasbourg appelée à être transférée en région parisienne. Ce plan s’inscrit dans un programme plus large annoncé en juillet 2020. Le groupe entend supprimer 1 700 emplois en Europe dont un millier en France sur trois ans. « Mais ce n’est qu’une partie du projet Pluton, prévient Jean-Louis Perrin, délégué CGT à Montpellier. Sanofi est en train de se désindustrialiser. Toute la pharmacie de synthèse est appelée à disparaître dans le groupe. Les sites de Sisteron, Elbeuf, Vertolaye, Brindisi (Italie), Francfort (Allemagne), Haverhill (Royaume-Uni), Újpest (Hongrie) sont destinés à sortir du groupe. Au total, cela représente 3 500 emplois. » Centre de distribution de Sanofi à Val-de-Reuil. -

Executive Profile: Genzyme's CEO on Dark Times, Transition and the Long
June 15th 2015 scripintelligence.com Executive profile: Genzyme’s CEO on dark times, transition and the long view David Meeker, CEO of Sanofi company Genzyme, recently found himself hustling back to Genzyme’s Kendall Square offices from a nearby meeting to talk to Scrip’s editor Eleanor Malone about the challenges and opportunities he has encountered in his varied career. In the first of a two-part series, he discusses his experiences in the company that he joined in 1994, and expounds upon issues faced by the industry as a whole. Eleanor Malone: Genzyme went through some dark times before the Sanofi acquisition with the manufacturing issues that led to shortages of enzyme replacement therapies. What lessons did you draw from those difficulties? David Meeker: You are absolutely correct; they were incredibly painful, dark moments. We failed to deliver on the implicit promise David Meeker addresses employees at of ensuring that patients can access the Genzyme’s HQ following a torch relay connecting the company’s sites near Boston medicines that they need. I think we learned to mark International Rare Diseases Day many lessons. On a very tactical level we learned about the lead time that it takes to bring biologic manufacturing up – we realised it right for the patient, given the limitations Many acquisitions destroy value, and a lot too late that we were not going to be at an we had. We were short of product so we had of the destruction happens when people adequate capacity and by the time we started to focus on how to manage the distribution leave: you have the products but you lose to build, even though it was several years of that product in a way which was most the know-how and the critical talent that ahead of when we ran out of medicine, we equitable. -

Fusiones 20De 20Labo
"Cuando los grandes se hacen gigantes" Fusiones de Laboratorios 1 INDICE PRÓLOGO……………………………………………………………………………...…….. 3 INTRODUCCIÓN: FUSIONES Y ADQUISICIONES…………………………………..….. 4 INVESTIGACIÓN Y DESARROLLO………………………………………………………. 5 LA INDUSTRIA FARMACEUTICA………………………………………………………. 16 FUSIÓN SANOFI – AVENTIS…………………………………………………………...… 20 FUSIÓN BAYER – SCHERING……………………………………………………………. 26 FUSIÓN PFIZER – WYETH……………………………………………………………….. 30 FUSIÓN MERCK & CO. – SCHERING PLOUGH…………………………………..……. 31 FUSIÓN ROCHE – GENENTECH………………………………………………………… 34 MERCADOS REGIONALES………………………………………………………….…… 40 LA INDUSTRIA FARMACÉUTICA EN LA ARGENTINA……………………...………. 42 CONCLUSIÓN……………………………………………………………………………… 49 2 PRÓLOGO Es un verdadero privilegio que hayan pensado en mí para prologar este interesante trabajo relacionado con la formación profesional de estos inquietos alumnos de la Carrera de Agentes de Propaganda Médica. Grato además, pues recrea mi participación activa en el mundo de la Industria Farmacéutica, en calidad de Asesor de la Fuerza de Ventas e Investigador Principal durante varias décadas, período que fue enriquecedor para mí y sumó un importante valor agregado a mi bagaje médico y personal. Entiendo que lo sucedido en los avances científicos y tecnológicos durante los últimos cuarenta años, ha constituido un quiebre en la Historia de la Medicina y, por lo tanto, de la Humanidad. Es por ello que veo con beneplácito que la formación de estos entusiastas jóvenes va de la mano con los cambios de planes y esquemas de estudios de los futuros médicos. -

The Life of the Abortion Pill in the United States
The Life of the Abortion Pill in the United States The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation The Life of the Abortion Pill in the United States (2000 Third Year Paper) Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:8852153 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA 80 The Life of the Abortion Pill in the United States Julie A. Hogan Eleven years after mifepristone1, the drug that chemically induces abortion and hence coined the abortion pill, was approved for use in France, American women still do not have access to the drug, although women in at least ten other nations do.2 In 1988, Americans thought the Abortion Pill [was] on the Hori- zon.3 In 1993, almost five years later, American women still did not have access to the drug, although many women's hopes were raised by newspaper headlines claiming that the Door May Be Open for [the] Abortion Pill to Be Sold in [the] U.S.4 and newspaper accounts predicting that mifepristone would be available in the United States in 1996.5 In 1996, the headlines reported that the Approval of [the] Abortion Pill by the FDA [was] Likely Soon.6 Yet, mifepristone was still not available in 1999, and newspaper headlines were less optimistic about pre- 1Mifepristone is the generic name for RU-486, the designation given the drug by its French maker, Roussel-Uclaf. -

Dyax Corp. 300 Technology Square Cambridge, MA 02139 617 225-2500
Dyax Corp. 300 Technology Square Cambridge, MA 02139 617 225-2500 www.dyax.com Other Offices Dyax SA, Liege, Belgium ADVANCING NOVEL THERAPEUTIC PRODUCTS Dyax Corp. Annual Report 2003 Corporate Information Dyax Achievements 2003 Dyax Goals 2004 Directors Executive Officers and Stock Listing Henry E. Blair Key Employees Common stock has been traded on the Nasdaq Stock Market Advances in Clinical Development Clinical Development Chairman, President and Henry E. Blair* under the symbol DYAX since our initial public offering in Milestones Chief Executive Officer, Chairman, President and August 14, 2000. DX-88/Hereditary Angioedema Dyax Corp. Chief Executive Officer DX-88/Hereditary Constantine E. Stephen S. Galliker, CPA* The following table gives the quarterly high and low sales G Completed 9-patient Phase II trial, met primary endpoints Angioedema Anagnostopoulos, Ph.D. EVP Finance and prices of our common stock for the last three years. G Genzyme Corporation joined Dyax as joint venture partner Managing General Partner, Administration and G Complete Phase II 2001 2002 2003 G Gateway Associates, LP Chief Financial Officer Completed 3 of 4 dose cohorts in 48-patient Phase II EDEMA1 trial EDEMA1 study High Low High Low High Low G Susan B. Bayh, J.D. Lynn G. Baird, Ph.D.* Initiated Phase II EDEMA2 trial G Periodically observe James W. Fordyce SVP Development First Quarter $20.94 $6.56 $11.38 $3.10 $2.25 $1.52 G Orphan Drug designation granted in U.S. and Europe effects of repeat dosing in Phase II EDEMA2 Managing Partner, Robert C. Ladner, Ph.D. Second Quarter $19.99 $6.81 $ 4.68 $3.20 $4.90 $1.67 Fordyce & Gabrielson, LLC SVP and Chief DX-88/On-Pump Open Heart Surgery (CABG) study Third Quarter $21.24 $6.05 $ 4.20 $1.65 $7.50 $2.58 Mary Ann Gray, Ph.D. -

The Story of RU-486 in the United States
The Story of RU-486 in the United States The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation The Story of RU-486 in the United States (2001 Third Year Paper) Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:8889480 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA TABLE OF CONTENTS INTRODUCTION 1 I. THE BIRTH OF A CONTROVERSY 2 A. Background: What is RU-486 and How Does It Work? 2 B. The French Abortion Pill Fury 3 II. POLITICS AND CHEMISTRY MAKE A VOLATILE MIXTURE: \KEEPING THE ABORTION PILL BOTTLED UP IN FRANCE" 7 A. Moral Concerns 7 A. Moral Concerns 7 B. Corporate America's Lack of Interest 8 B. Corporate America's Lack of Interest 8 C. Supreme Court Jurisprudence's Impact on RU-486 10 D. Abortion Politics Goes Conservative 13 E. Regulatory Restrictions on Abortion 13 III. THE ABORTION TIDE BEGINS TO TURN 17 A. A New Administration 17 B. The Involvement of the Population Council 19 C. Concern From the Pro-Life Movement 22 1 IV. THE TIDE TURNS AGAIN { The Political Pendulum Swings to the Right 25 A. The Politics of Abortion Run Into The Contract With America 25 B. Conservative Congressional Legislation 27 V. THE FDA APPROVAL PROCESS GETS UNDERWAY 29 V. -

Cerezyme, INN-Imiglucerase
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Cerezyme 400 Units Powder for concentrate for solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial contains 400 units* of imiglucerase**. After reconstitution, the solution contains 40 units (approximately 1.0 mg) of imiglucerase per ml (400 U/10 ml). * An enzyme unit (U) is defined as the amount of enzyme that catalyses the hydrolysis of one micromole of the synthetic substrate para-nitrophenyl β-D-glucopyranoside (pNP-Glc) per minute at 37°C. ** Imiglucerase is a modified form of human acid β-glucosidase and is produced by recombinant DNA technology using a mammalian Chinese Hamster Ovary (CHO) cell culture, with mannose modification for targeting macrophages. Excipients: For the full list of excipients, see section 6.1. This medicinal product contains sodium and is administered in 0.9% sodium chloride intravenous solution (see section 6.6). After reconstitution, the solution contains 1.24 mmol sodium (400 U/10 mL). To be taken into consideration by patients on a controlled sodium diet. 3. PHARMACEUTICAL FORM Powder for concentrate for solution for infusion. Cerezyme is a white to off-white powder. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Cerezyme (imiglucerase) is indicated for use as long-term enzyme replacement therapy in patients with a confirmed diagnosis of non-neuronopathic (Type 1) or chronic neuronopathic (Type 3) Gaucher disease who exhibit clinically significant non-neurological manifestations of the disease. The non-neurological manifestations of Gaucher disease include one or more of the following conditions: • anaemia after exclusion of other causes, such as iron deficiency • thrombocytopenia • bone disease after exclusion of other causes such as Vitamin D deficiency • hepatomegaly or splenomegaly 4.2 Posology and method of administration Disease management should be directed by physicians knowledgeable in the treatment of Gaucher disease. -

Opemmundi E Ljbope
OpemMundi E lJBOPE A WEEKLY REPORT ON THE ECONOMY OF THE COMMON MARKET ----- ------- -- �--- --· ------ -- - -----·-----·--·-------- of ooo oo oo 0000000000 ��-ONTENTt-4.oo o_Q';)o o oq ooo oo 0000001 g�!. ·----- ---·--····-············· ······-----· __ :__ --- ------ - - - . : g 0 I 0 o I : o / o I : o o I : o 0g I /ffJ ;1 r'i\J : og o INDEX f .i trr rtn "� o _ _, ;.;,·� Jt\J T 0 '-"' 1.•'- l",, 0 , ...4 �� ..1' ., . 0 ft iii 0 0 0 0 JO 0 I 0 0 0 0o To Euroflash 0o 0 0 0 0 0 0 ,0 0 , g from No 468 to No 492 inclusive g .,0 0 !o o 1 o o 0 0 0 0 0 o July 4, 1968 - December 19 , 1963 °o 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 · t;tlllOFLA.SH: llusiness 11enetration U('ross Europe o 01 0 0 :o g January 1969 Index No 11 · l g 0 :o 0 ,o 0 :o O :o 0 :o 0 :o 01 : 0 0 0 ooo -----------------------------------------------------o o oo o oo o oo oo oo o o_o oo o oo_o o o o o== oo--=:�� o o------o o o=:o -------------- oo o o oo oo �=� o-----o o o o Opera Mundi ElfR OPE A WEEKLY REPORT ON THE ECONOMY OF THE COMMON MARKET PUBLISHED ON BEHALF OF OPERA MUNDI BY EUROPEAN INTELLIGENCE LIMITED EUROPA HOUSE ROYAL TUNBRIDGE WELLS KENT TEL 25202/4 TELEX 95114 OPERA MUNDI EUROPE 100 Avenue Raymond Poincar� - PARIS 16e TEL: KLE 54-12 34-21 - CCP PARIS 3235-50 EDITOR & PUBLISHER . -
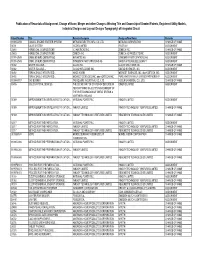
Publication of Recordals of Assignment, Change of Name
Publication of Recordals of Assignment, Change of Name, Merger and other Changes Affecting Title and Ownership of Granted Patents, Registered Utility Models, Industrial Designs and Lay-out Designs Topography) of Integrated Circuit Patent Number Title Patentee/Assignor Assignee/New Name Remarks 1199654059 COAXIAL ENGINE STARTER SYSTEM MITSUBA ELECTRIC MFG. CO. LTD. MITSUBA CORPORATION CHANGE OF NAME 31731 VALVE SYSTEM ISCOR LIMITED IPCOR NV ASSIGNMENT 25483 HERBICIDAL COMPOSITIONS ICI AMERICAS INC. ZENECA INC. CHANGE OF NAME 25483 HERBICIDAL COMPOSITIONS ZENECA INC. ZENECA AG PRODUCTS INC. ASSIGNMENT 1199142549 OXIME ETHERS DERIVATIVES NOVARTIS AG SYNGENTA PARTICIPATIONS AG ASSIGNMENT 1199142549 OXIME ETHERS DERIVATIVES SYNGENTA PARTICIPATIONS AG BAYER AKTIENGESELLSCHAFT ASSIGNMENT 31882 WATER SOLUBLE… GLAXO INC. GLAXO WELLCOME INC. CHANGE OF NAME 31882 WATER SOLUBLE… GLAXO WELLCOME INC. GILEAD SCIENCES, INC. ASSIGNMENT 31492 REPLACEABLE INTEGRATED… AMOS KORIN MIDWEST SCIENCES, INC. dba KORTECH, INC. ASSIGNMENT 31492 REPLACEABLE INTEGRATED… MIDWEST SCIENCES, INC. dba KORTECH, INC. PURE WATER FAMILY LIMITED PARTNERSHIP ASSIGNMENT 1199447817 GAS BURNER TRI-SQUARE INDUSTRIAL CO., LTD. HOSUN UNIVERSAL CO., LTD. CHANGE OF NAME 18495 LIQUID CRYSTAL DEVICES THE SECRETARY OF STATE FOR DEFENSE IN QINETIQ LIMITED ASSIGNMENT HER BRITTANIC MAJESTY'S GOVERNMENT OF THE UNITED KINGDOM OF GREAT BRITAIN & NORTHERN IRELAND 31389 IMPROVEMENTS IN OR RELATING TO CATION… NATIONAL POWER PLC INNOGY LIMITED ASSIGNMENT 31389 IMPROVEMENTS IN OR RELATING TO CATION… -

2017 Annual Report on Form
FORM 20-F 2017 UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 20-F (Mark One) ‘ REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934 or È ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 2017 Or ‘ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 Or ‘ SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 Date of event requiring this shell company report For the transition period from to Commission File Number: 001-31368 Sanofi (Exact name of registrant as specified in its charter) N/A (Translation of registrant’s name into English) France (Jurisdiction of incorporation or organization) 54, Rue La Boétie, 75008 Paris, France (Address of principal executive offices) Karen Linehan, Executive Vice President Legal Affairs and General Counsel 54, Rue La Boétie, 75008 Paris, France. Fax: 011 + 33 1 53 77 43 03. Tel: 011 + 33 1 53 77 40 00 (Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person) Securities registered or to be registered pursuant to Section 12(b) of the Act: Title of each class: Name of each exchange on which registered: American Depositary Shares, each representing one half of one ordinary share, par value €2 per share New York Stock Exchange Ordinary shares, par value €2 per share New York Stock Exchange (for listing purposes only) Contingent Value Rights NASDAQ Global Market Securities registered pursuant to Section 12(g) of the Act: None The number of outstanding shares of each of the issuer’s classes of capital or common stock as of December 31, 2017 was: Ordinary shares: 1,254,019,904 Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.