Dyax Corp. 300 Technology Square Cambridge, MA 02139 617 225-2500
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Scientific Organizing Committee Gratefully Acknowledges the Pharmaceutical and Biotechnology Industry for Their Generous Support of WCBP 2019
The Scientific Organizing Committee gratefully acknowledges the pharmaceutical and biotechnology industry for their generous support of WCBP 2019: Strategic Diamond Program Partners F. Hoffmann-La Roche Ltd. Genentech, a Member of the Roche Group Strategic Platinum Program Partners Amgen Inc. Biogen MedImmune, A member of the AstraZeneca Group Strategic Gold Program Partners Eli Lilly and Company Merck & Co., Inc. Novo Nordisk A/S Pfizer, Inc. Strategic Silver Program Partner Sanofi 1 Gold Program Partner Bill and Melinda Gates Foundation Silver Program Partners BioMarin Pharmaceutical Inc. Bristol-Myers Squibb Company GlaxoSmithKline Jazz Pharmaceuticals Bronze Program Partner Seattle Genetics, Inc. Friend of CASSS Janssen R&D, LLC 2 The Scientific Organizing Committee gratefully acknowledges the Program Partners and Exhibitors for their generous support of WCBP 2019: Diamond Program Partners Agilent Technologies Catalent Pharma Solutions Eurofins Pharma Discovery Services ProteinSimple, a Bio-Techne brand Thermo Fisher Scientific Waters Corporation Platinum Program Partners BioAnalytix SCIEX Bronze Program Partner Bruker Corporation 3 The Scientific Organizing Committee gratefully acknowledges the Exhibitors for their generous support of WCBP 2019: Exhibitor Partners 908 Devices Inc. NanoImaging Services Agilent Technologies New England Biolabs Associates of Cape Cod, Inc. Postnova Analytics Inc. BioAnalytix Inc. Pressure BioSciences Inc. Bruker Corporation Protein Metrics, Inc. Catalent Pharma Solutions ProteinSimple, a Bio-Techne brand Charles River Laboratories ProZyme, A part of Agilent Covance, Inc. RedShift BioAnalytics, Inc. Cygnus Technologies Rockland Immunochemicals, Inc. Envigo SCIEX Eurofins BioPharma Product Testing SGS Life Science Services Eurofins Pharma Discovery Services Shimadzu Scientific Instruments, Inc. FortéBio - Biologics by Molecular Devices Thermo Fisher Scientific GE Healthcare Life Sciences U.S. -

The Impact of Secondary Innovation on Firm Market Value in the Pharmaceutical Industry
The Impact of Secondary Innovation on Firm Market Value in the Pharmaceutical Industry By: Maitri Punjabi Honors Thesis Economics Department The University of North Carolina at Chapel Hill March 2016 Approved: ______________________________ Dr. Jonathan Williams Punjabi 2 Abstract This paper analyzes the effect of the changing nature of innovation on pharmaceutical firm market value from the years 1987 to 2010 by using U.S. patent and claim data. Over the years, firms have started shifting focus from primary innovation to secondary innovation as new ideas and new compounds become more difficult to generate. In this study, we analyze the impact of this patent portfolio shift on the market capitalization of pharmaceutical firms. After using firm fixed effects and the instrumental variable approach, we find that there exists a strong positive relationship between secondary innovations and the market value of the firm– in fact, we find a stronger relationship than is observed between primary innovation and market value. When focusing on the different levels of innovation within the industry, we find that this relationship is stronger for less-innovative firms (those that have produced fewer patents) than it is for highly- innovative firms. We also find that this relationship is stronger for firms that spend less on research and development, complementing earlier findings that research productivity is declining over time. Punjabi 3 Acknowledgements I would primarily like to thank my adviser, Dr. Jonathan Williams, for his patience and constant support. Without his kind and helpful attitude, this project would have been a much more frustrating process. Through his knowledge of the industry, I have gained valuable insight and have learned a great deal about a unique and growing field. -

United States Securities and Exchange Commission Form 10-K Shire Pharmaceuticals Group
UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 10-K (Mark One) ፤ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 1999 អ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 Commission file number 0-29630 SHIRE PHARMACEUTICALS GROUP PLC (Exact name of registrant as specified in its charter) England and Wales (State or other jurisdiction (I.R.S. Employer of incorporation or organization) Identification No.) N.A. East Anton, Andover, Hampshire SP10 5RG England (Address of principal executive offices) (Zip Code) 44 1264 333455 (Registrant's telephone number, including area code) Securities registered pursuant to Section 12(b) of the Act: Title of each class Name of exchange on which registered American Depository Shares, each representing Nasdaq National Market 3 Ordinary Shares, 5 pence nominal value per share Securities registered pursuant to Section 12(g) of the Act: None (Title of class) Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ፤ No អ Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the Registrant's knowledge, in definitive proxy or information statements incorporated by reference to Part III of this Form 10-K or any amendment to this Form 10-K. -

National Lipid Association Annual Summary of Clinical Lipidology 2016 Disclosures Dr. Harold E. Bays: Dr. Bays Discloses That He
National Lipid Association Annual Summary of Clinical Lipidology 2016 Disclosures Dr. Harold E. Bays: Dr. Bays discloses that he has received research grants from Arena Pharmaceuticals, Boehringer Ingelheim, Cargill Inc., GlaxoSmithKline, Novo Nordisk, Orexigen Therapeutics, Shionogi, Takeda, Stratum Nutrition, California Raisin Board, Esperion, Essentialis, Forest, Gilead Sciences Inc., Given, Hoffman-LaRoche, Home Access, Novartis, Omthera, Pfizer, Trygg Pharmaceuticals, TWI Bio, Xoma, Ardea Inc., High Point Pharmaceuticals LLC, Micropharma Limited, TransTech Pharma Inc., TIMI, Pozen, Regeneron, and Elcelyx. He further discloses that he has received honoraria/research grants from Amarin, Amgen, AstraZeneca, Bristol-Myers Squibb, Catabasis, Daiichi-Sankyo Inc., Eisai, Merck & Co, VIVUS, Zeomedex, and WPU. Dr. Peter H. Jones: Dr. Jones has received consulting or speaker honoraria from Merck and Co., Amgen, and Sanofi-Aventis/Regeneron. Dr. W. Virgil Brown: Dr. Brown is the editor of the Journal of Clinical Lipidology and further discloses that he has received consulting fees/honoraria from Akcea, Esperion, Regeneron, Amgen, Genzyme, Pfizer Inc., Merck and Co, GlaxoSmithKline, Medtelligence, and Vindico. Dr. Terry A. Jacobson: Dr. Jacobson has received consulting fees from Merck and Co., Amarin, Amgen, AstraZeneca, and Regeneron/Sanofi-Aventis. Dr. Karen E. Aspry: Dr. Aspry has no disclosures to report. Dr. Christie M. Ballantyne: Dr. Ballantyne has received research grants from Abbott Diagnostics, Amarin, Amgen, Eli Lilly, Esperion, ISIS Pharmaceuticals, Novartis Pharmaceuticals, Pfizer, Otsuka, Regeneron, Roche Diagnostic, Sanofi-Synthelabo, and Takeda Pharmaceuticals. He has also received consulting fees from Abbott Diagnostics, Amarin, Amgen, AstraZeneca, Eli Lilly, Esperion, Genzyme, ISIS Pharmaceuticals, Matinas BioPharma Inc., Merck and Co., Novartis Pharmaceuticals, Pfizer, Regeneron, Roche, and Sanofi-Synthelabo. -

Executive Profile: Genzyme's CEO on Dark Times, Transition and the Long
June 15th 2015 scripintelligence.com Executive profile: Genzyme’s CEO on dark times, transition and the long view David Meeker, CEO of Sanofi company Genzyme, recently found himself hustling back to Genzyme’s Kendall Square offices from a nearby meeting to talk to Scrip’s editor Eleanor Malone about the challenges and opportunities he has encountered in his varied career. In the first of a two-part series, he discusses his experiences in the company that he joined in 1994, and expounds upon issues faced by the industry as a whole. Eleanor Malone: Genzyme went through some dark times before the Sanofi acquisition with the manufacturing issues that led to shortages of enzyme replacement therapies. What lessons did you draw from those difficulties? David Meeker: You are absolutely correct; they were incredibly painful, dark moments. We failed to deliver on the implicit promise David Meeker addresses employees at of ensuring that patients can access the Genzyme’s HQ following a torch relay connecting the company’s sites near Boston medicines that they need. I think we learned to mark International Rare Diseases Day many lessons. On a very tactical level we learned about the lead time that it takes to bring biologic manufacturing up – we realised it right for the patient, given the limitations Many acquisitions destroy value, and a lot too late that we were not going to be at an we had. We were short of product so we had of the destruction happens when people adequate capacity and by the time we started to focus on how to manage the distribution leave: you have the products but you lose to build, even though it was several years of that product in a way which was most the know-how and the critical talent that ahead of when we ran out of medicine, we equitable. -

2016 Medicines in Development for Rare Diseases a LIST of ORPHAN DRUGS in the PIPELINE
2016 Medicines in Development for Rare Diseases A LIST OF ORPHAN DRUGS IN THE PIPELINE Autoimmune Diseases Product Name Sponsor Official FDA Designation* Development Status Actemra® Genentech treatment of systemic sclerosis Phase III tocilizumab South San Francisco, CA www.gene.com Adempas® Bayer HealthCare Pharmaceuticals treatment of systemic sclerosis Phase II riociguat Whippany, NJ www.pharma.bayer.com ARA 290 Araim Pharmaceuticals treatment of neuropathic pain in patients Phase II Tarrytown, NY with sarcoidosis www.ariampharma.com ARG201 arGentis Pharmaceuticals treatment of diffuse systemic sclerosis Phase II (type 1 native bovine skin Collierville, TN www.argentisrx.com collagen) BYM338 Novartis Pharmaceuticals treatment of inclusion body myositis Phase III (bimagrumab) East Hanover, NJ www.novartis.com CCX168 ChemoCentryx treatment of anti-neutrophil cytoplasmic Phase II (5a receptor antagonist) Mountain View, CA auto-antibodies associated vasculitides www.chemocentryx.com (granulomatosis with polyangitis or Wegener's granulomatosis), microscopic polyangitis, and Churg-Strauss syndrome * This designation is issued by the FDA's Office of Orphan Products Development while the drug is still in development. The designation makes the sponsor of the drug eligible for entitlements under the Orphan Drug Act of 1983. The entitlements include seven years of marketing exclusivity following FDA approval of the drug for the designated use. Medicines in Development: Rare Diseases | 2016 1 Autoimmune Diseases Product Name Sponsor Official FDA -

Cerezyme, INN-Imiglucerase
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Cerezyme 400 Units Powder for concentrate for solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial contains 400 units* of imiglucerase**. After reconstitution, the solution contains 40 units (approximately 1.0 mg) of imiglucerase per ml (400 U/10 ml). * An enzyme unit (U) is defined as the amount of enzyme that catalyses the hydrolysis of one micromole of the synthetic substrate para-nitrophenyl β-D-glucopyranoside (pNP-Glc) per minute at 37°C. ** Imiglucerase is a modified form of human acid β-glucosidase and is produced by recombinant DNA technology using a mammalian Chinese Hamster Ovary (CHO) cell culture, with mannose modification for targeting macrophages. Excipients: For the full list of excipients, see section 6.1. This medicinal product contains sodium and is administered in 0.9% sodium chloride intravenous solution (see section 6.6). After reconstitution, the solution contains 1.24 mmol sodium (400 U/10 mL). To be taken into consideration by patients on a controlled sodium diet. 3. PHARMACEUTICAL FORM Powder for concentrate for solution for infusion. Cerezyme is a white to off-white powder. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Cerezyme (imiglucerase) is indicated for use as long-term enzyme replacement therapy in patients with a confirmed diagnosis of non-neuronopathic (Type 1) or chronic neuronopathic (Type 3) Gaucher disease who exhibit clinically significant non-neurological manifestations of the disease. The non-neurological manifestations of Gaucher disease include one or more of the following conditions: • anaemia after exclusion of other causes, such as iron deficiency • thrombocytopenia • bone disease after exclusion of other causes such as Vitamin D deficiency • hepatomegaly or splenomegaly 4.2 Posology and method of administration Disease management should be directed by physicians knowledgeable in the treatment of Gaucher disease. -
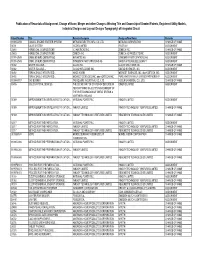
Publication of Recordals of Assignment, Change of Name
Publication of Recordals of Assignment, Change of Name, Merger and other Changes Affecting Title and Ownership of Granted Patents, Registered Utility Models, Industrial Designs and Lay-out Designs Topography) of Integrated Circuit Patent Number Title Patentee/Assignor Assignee/New Name Remarks 1199654059 COAXIAL ENGINE STARTER SYSTEM MITSUBA ELECTRIC MFG. CO. LTD. MITSUBA CORPORATION CHANGE OF NAME 31731 VALVE SYSTEM ISCOR LIMITED IPCOR NV ASSIGNMENT 25483 HERBICIDAL COMPOSITIONS ICI AMERICAS INC. ZENECA INC. CHANGE OF NAME 25483 HERBICIDAL COMPOSITIONS ZENECA INC. ZENECA AG PRODUCTS INC. ASSIGNMENT 1199142549 OXIME ETHERS DERIVATIVES NOVARTIS AG SYNGENTA PARTICIPATIONS AG ASSIGNMENT 1199142549 OXIME ETHERS DERIVATIVES SYNGENTA PARTICIPATIONS AG BAYER AKTIENGESELLSCHAFT ASSIGNMENT 31882 WATER SOLUBLE… GLAXO INC. GLAXO WELLCOME INC. CHANGE OF NAME 31882 WATER SOLUBLE… GLAXO WELLCOME INC. GILEAD SCIENCES, INC. ASSIGNMENT 31492 REPLACEABLE INTEGRATED… AMOS KORIN MIDWEST SCIENCES, INC. dba KORTECH, INC. ASSIGNMENT 31492 REPLACEABLE INTEGRATED… MIDWEST SCIENCES, INC. dba KORTECH, INC. PURE WATER FAMILY LIMITED PARTNERSHIP ASSIGNMENT 1199447817 GAS BURNER TRI-SQUARE INDUSTRIAL CO., LTD. HOSUN UNIVERSAL CO., LTD. CHANGE OF NAME 18495 LIQUID CRYSTAL DEVICES THE SECRETARY OF STATE FOR DEFENSE IN QINETIQ LIMITED ASSIGNMENT HER BRITTANIC MAJESTY'S GOVERNMENT OF THE UNITED KINGDOM OF GREAT BRITAIN & NORTHERN IRELAND 31389 IMPROVEMENTS IN OR RELATING TO CATION… NATIONAL POWER PLC INNOGY LIMITED ASSIGNMENT 31389 IMPROVEMENTS IN OR RELATING TO CATION… -

An Emerging National Resource for Clinical Research and Your Healthcare Partner in Personalized Medicine Innovation
An Exclusive Membership Look into Colorado To visualize improving the world around us Fisher Scientifi c, part of Thermo Fisher Scientifi c, the world leader in serving science, is pleased to have the opportunity to partner with the Colorado BioScience Association and act as primary supplier of laboratory products, safety supplies, equipment, chemicals, reagents and a host of services. Fisher Scientifi c understands today’s challenging environment and has capabilities that align with CBSA’s strategic goals going beyond just providing products to o ering Colorado BioScience Association members access to customized programs to fi t their specifi c needs from start-up to scale-up. 1.800.766.7000 • Fax: 1.800.926.1166 • www.fi shersci.com 2 BIOSCIENCECOLORADO 2014-2015 Litho in U.S.A. 13_0280 JJ/KJ COLOR ADO 2014-2015 Drawing People & Data Together to Benefit Human Health 10 22 26 14 6 CONTENTS 06 Mobile Health in Colorado: Where “Big Data” Sparks Big Ideas 10 Biodesix Harnesses Machine Learning and Analytics to Tackle Multivariate Diagnostics 14 Bioscience in Colorado Continues to Shine with U.S. Senator Michael Bennet 16 Color ado Life Science Assets: A Sophisticated Repertoire of Clinical-Stage Therapeutic, Device and Diagnostic Products 22 Webb-Waring Biomedical Research Program 26 F ound in Translation: Rocky Mountain Lions Eye Institute Translational Research Programs Focus on Innovative Treatment of Eye Diseases 30 Connecting the Dots: Tech Transfer Offices Connect Research and Marketplace to Stimulate the State's Economy 33 Colorado by the Numbers: Financing and Acquisitions 36 Grant Programs: Innovation Engines 48 Colorado Bioscience Industry Directory 2014-2015 BIOSCIENCECOLORADO 3 We Know Workforce elcome to the Colorado BioScience Association’s eleventh anniversary edition of Bioscience Colorado. -

Immunocore and Medimmune Announce New Collaboration to Conduct Immuno-Oncology Combination Trials in Melanoma
PRESS RELEASE – IMMUNOCORE LIMITED Immunocore and MedImmune announce new collaboration to conduct immuno-oncology combination trials in melanoma (Oxford, UK, 16 April 2015) Immunocore Limited, a world-leading biotechnology company developing novel biological drugs to treat cancer and other diseases, and MedImmune, the global biologics research and development arm of AstraZeneca, today announced that they have entered into a second collaboration. Under the terms of the agreement, Immunocore will conduct a Phase Ib/II clinical trial combining MedImmune’s investigational checkpoint inhibitors MEDI4736 (anti-PD-L1) and/or tremelimumab (anti-CTLA-4) with IMCgp100, Immunocore’s lead T-cell receptor based investigational therapeutic, for the potential treatment of patients with metastatic melanoma. MedImmune has an exclusive relationship with Immunocore for the development of IMCgp100 in combination with MEDI4736 and/or tremelimumab, and will have first right of negotiation for the future commercial development of these combinations for tumours expressing gp100. Immunocore and MedImmune will collaborate to establish a dosing regimen for IMCgp100 combined with MEDI4736 and/or tremelimumab, as part of the Phase Ib study. The Phase II study will assess the safety and efficacy of the different combinations. The companies have a pre-existing research collaboration and licensing agreement, announced in January 2014, to develop novel cancer therapies using Immunocore’s Immune Mobilising Monoclonal T-Cell Receptor Against Cancer (ImmTAC) technology. “We are pleased to expand our partnership with Immunocore, a leader in the discovery and development of novel T-cell receptor-based drugs, to include this combination clinical trial in melanoma,” said Dr. Ed Bradley, Senior Vice President and Head of the Oncology Innovative Medicines unit, MedImmune. -

Summit for Clinical Ops Executives
Organized by Register Today! FINAL AGENDA 7th Annual February 23 - 25, 2016 | Hyatt Regency Miami, Miami, FL Back in SUMMIT FOR CLINICAL OPS EXECUTIVES Miami! February 23-24 February 24-25 Global Site Selection, Feasibility Assessment, Improving Site-Study Activation and Performance Operations and Site Management Patient Engagement, Enrollment and Retention through Enrollment Planning and Patient Recruitment Communities and Tech Clinical Trial Forecasting and Budgeting Managing Outsourced Clinical Trials Implementing Risk-Based Monitoring (Part 1) Implementing Risk-Based Monitoring (Part 2) Clinical IT Strategy and Governance Clinical Data Technology and Integration Managing Late Stage Research, Observational Leveraging Existing Data for Clinical and Studies and Registries Observational Research Symposia - February 23-24 Managing Biomarker-Driven Clinical Trials NEW Clinical Research Statistics for Non-Statisticians NEW Event Features BEST 12 Conferences Clinical Informatics News Best Practices Awards P R S 2 Plenary Keynote Sessions Dedicated Exhibit Hall Hours & A E C T I C Networking Functions 3 Short Courses RAC RAC RAC P T P T P T T IC T IC T IC S E S E S E Interactive Breakout Discussions 2 New Symposia E S E S E S B B B 2 n 0 1 o i 2 2 4 t 0 E 0 R H n 1,000+ Industry Leaders Expected in 2016 1 E o e 1 E 4SCOPEsummit.comn M 4 N W I N N o r a b l e N O M I PREMIER SPONSORS Event-at-a-Glance Monday, February 22, 2016 Pre-Conference Short Courses (Optional, Separate Registration Required) 1. -

The Future of Biomanufacturing
THE FUTURE OF BIOMANUFACTURING WELCOME TO A PLACE THAT DELIVERS. HEADER 2 | BIOMANUFACTURING WELCOME TO EUROPE’S MOST SIGNIFICANT CLUSTER OF BIOMANUFACTURING. Liverpool City Region is home to the world’s biggest pharmaceutical players. It is also one of the world’s longest established biomanufacturing centres, chosen by the UK government in the 1940s for the largest penicillin manufacturing plant in the world. Major companies continue to build on this long-tradition and reinvest in new world-class facilities. Our formula for pharma manufacturing success is clear. Liverpool City Region sits at the centre of the UK’s chemical engineering industry; is part of a major centre for health and life sciences and is driving the advanced manufacturing sector through digital innovation. The convergence of these assets has a catalytic impact on the availability of the science, technology and engineering talent that underpins biomanufacturing. Most importantly though, a committed partnership of local organisations is supporting existing manufacturers and the wider supply-chain, through programmes to invest in the infrastructure, facilities and skills that the sector needs to succeed. Welcome to your future-proofed business location. BIOMANUFACTURING | 3 M65 M 6 SEFTON M 6 2 6 6 M M 6 1 M58 62 M M 6 GLASGOW M602 EDINBURGH M MANCHESTER 5 7 M 6 GLOBAL 0 TRADE KNOSLEY ROUTES ST. HELENS 0 6 M NEWCASTLE LIVERPOOL M 62 6 15 M 10 11 M62 12 14 13 HALTON LIVERPOOL CITY REGION LEEDS 56 MANCHESTER HULL M 1 AIRPORT MANCHESTER 7 RIVER 4 M MERSEY 2 9 5 8 SHEFFIELD IRRAL 5 3 3 M LIVERPOOL JOHN 6 LENNON AIRPORT NOTTINGHAM AL CAN 6 SHIP STER CHE MAN BIRMINGHAM 6 M5 CARDIFF BRISTOL LONDON PLYMOUTH SOUTHAMPTON 4 | BIOMANUFACTURING M65 M 6 SEFTON M 6 2 6 6 M M 6 1 M58 62 M M 6 M602 M MANCHESTER 5 7 M 6 GLOBAL 0 TRADE KNOSLEY ROUTES ST.