Cerezyme, INN-Imiglucerase
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Intravenous Enzyme Replacement Therapy (ERT) for Gaucher Disease
UnitedHealthcare® Commercial Medical Benefit Drug Policy Intravenous Enzyme Replacement Therapy (ERT) for Gaucher Disease Policy Number: 2021D0048K Effective Date: April 1, 2021 Instructions for Use Table of Contents Page Related Commercial Policy Coverage Rationale ....................................................................... 1 • Provider Administered Drugs – Site of Care Applicable Codes .......................................................................... 3 Background.................................................................................... 3 Community Plan Policy Benefit Considerations .................................................................. 3 • Intravenous Enzyme Replacement Therapy (ERT) Clinical Evidence ........................................................................... 4 for Gaucher Disease U.S. Food and Drug Administration ............................................. 6 Centers for Medicare and Medicaid Services ............................. 6 References ..................................................................................... 7 Policy History/Revision Information ............................................. 8 Instructions for Use ....................................................................... 8 Coverage Rationale See Benefit Considerations This policy refers to the following drug products, all of which are intravenous enzyme replacement therapies used in the treatment of Gaucher disease: Cerezyme® (imiglucerase) Elelyso® (taliglucerase) VPRIV® (velaglucerase) -

Vpriv-Pm-En.Pdf
PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION PrVPRIV® velaglucerase alfa Powder for Solution for Injection 400 U/vial, Intravenous Enzyme Replacement Therapy ATC code: A16AB10 Takeda Canada Inc. Date of Initial Approval: 22 Adelaide Street West, Suite 3800 October 1, 2010 Toronto Ontario M5H 4E3 Date of Revision: December 21, 2020 Submission Control No: 241844 VPRIV® and the VPRIV Logo® are registered trademarks of Shire Human Genetic Therapies, Inc. TAKEDA™ and the TAKEDA Logo® are trademarks of Takeda Pharmaceutical Company Limited, used under license. Page 1 of 24 RECENT MAJOR LABEL CHANGES Warnings and Precautions, Immunogenicity (8) 9/2020 Warnings and Precautions, Infusion-Related Reactions (8) 9/2020 Warnings and Precautions, Breast-feeding (8.1.2) 9/2020 TABLE OF CONTENTS PART I: HEALTH PROFESSIONAL INFORMATION ................................................................. 4 1 INDICATIONS .................................................................................................................4 2 CONTRAINDICATIONS ..................................................................................................4 4 DOSAGE AND ADMINISTRATION ................................................................................ 4 4.1 Dosing Considerations ..........................................................................................4 4.2 Recommended Dose and Dosage Adjustment ....................................................... 4 4.3 Administration .......................................................................................................4 -

National Lipid Association Annual Summary of Clinical Lipidology 2016 Disclosures Dr. Harold E. Bays: Dr. Bays Discloses That He
National Lipid Association Annual Summary of Clinical Lipidology 2016 Disclosures Dr. Harold E. Bays: Dr. Bays discloses that he has received research grants from Arena Pharmaceuticals, Boehringer Ingelheim, Cargill Inc., GlaxoSmithKline, Novo Nordisk, Orexigen Therapeutics, Shionogi, Takeda, Stratum Nutrition, California Raisin Board, Esperion, Essentialis, Forest, Gilead Sciences Inc., Given, Hoffman-LaRoche, Home Access, Novartis, Omthera, Pfizer, Trygg Pharmaceuticals, TWI Bio, Xoma, Ardea Inc., High Point Pharmaceuticals LLC, Micropharma Limited, TransTech Pharma Inc., TIMI, Pozen, Regeneron, and Elcelyx. He further discloses that he has received honoraria/research grants from Amarin, Amgen, AstraZeneca, Bristol-Myers Squibb, Catabasis, Daiichi-Sankyo Inc., Eisai, Merck & Co, VIVUS, Zeomedex, and WPU. Dr. Peter H. Jones: Dr. Jones has received consulting or speaker honoraria from Merck and Co., Amgen, and Sanofi-Aventis/Regeneron. Dr. W. Virgil Brown: Dr. Brown is the editor of the Journal of Clinical Lipidology and further discloses that he has received consulting fees/honoraria from Akcea, Esperion, Regeneron, Amgen, Genzyme, Pfizer Inc., Merck and Co, GlaxoSmithKline, Medtelligence, and Vindico. Dr. Terry A. Jacobson: Dr. Jacobson has received consulting fees from Merck and Co., Amarin, Amgen, AstraZeneca, and Regeneron/Sanofi-Aventis. Dr. Karen E. Aspry: Dr. Aspry has no disclosures to report. Dr. Christie M. Ballantyne: Dr. Ballantyne has received research grants from Abbott Diagnostics, Amarin, Amgen, Eli Lilly, Esperion, ISIS Pharmaceuticals, Novartis Pharmaceuticals, Pfizer, Otsuka, Regeneron, Roche Diagnostic, Sanofi-Synthelabo, and Takeda Pharmaceuticals. He has also received consulting fees from Abbott Diagnostics, Amarin, Amgen, AstraZeneca, Eli Lilly, Esperion, Genzyme, ISIS Pharmaceuticals, Matinas BioPharma Inc., Merck and Co., Novartis Pharmaceuticals, Pfizer, Regeneron, Roche, and Sanofi-Synthelabo. -

Executive Profile: Genzyme's CEO on Dark Times, Transition and the Long
June 15th 2015 scripintelligence.com Executive profile: Genzyme’s CEO on dark times, transition and the long view David Meeker, CEO of Sanofi company Genzyme, recently found himself hustling back to Genzyme’s Kendall Square offices from a nearby meeting to talk to Scrip’s editor Eleanor Malone about the challenges and opportunities he has encountered in his varied career. In the first of a two-part series, he discusses his experiences in the company that he joined in 1994, and expounds upon issues faced by the industry as a whole. Eleanor Malone: Genzyme went through some dark times before the Sanofi acquisition with the manufacturing issues that led to shortages of enzyme replacement therapies. What lessons did you draw from those difficulties? David Meeker: You are absolutely correct; they were incredibly painful, dark moments. We failed to deliver on the implicit promise David Meeker addresses employees at of ensuring that patients can access the Genzyme’s HQ following a torch relay connecting the company’s sites near Boston medicines that they need. I think we learned to mark International Rare Diseases Day many lessons. On a very tactical level we learned about the lead time that it takes to bring biologic manufacturing up – we realised it right for the patient, given the limitations Many acquisitions destroy value, and a lot too late that we were not going to be at an we had. We were short of product so we had of the destruction happens when people adequate capacity and by the time we started to focus on how to manage the distribution leave: you have the products but you lose to build, even though it was several years of that product in a way which was most the know-how and the critical talent that ahead of when we ran out of medicine, we equitable. -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole -

Dyax Corp. 300 Technology Square Cambridge, MA 02139 617 225-2500
Dyax Corp. 300 Technology Square Cambridge, MA 02139 617 225-2500 www.dyax.com Other Offices Dyax SA, Liege, Belgium ADVANCING NOVEL THERAPEUTIC PRODUCTS Dyax Corp. Annual Report 2003 Corporate Information Dyax Achievements 2003 Dyax Goals 2004 Directors Executive Officers and Stock Listing Henry E. Blair Key Employees Common stock has been traded on the Nasdaq Stock Market Advances in Clinical Development Clinical Development Chairman, President and Henry E. Blair* under the symbol DYAX since our initial public offering in Milestones Chief Executive Officer, Chairman, President and August 14, 2000. DX-88/Hereditary Angioedema Dyax Corp. Chief Executive Officer DX-88/Hereditary Constantine E. Stephen S. Galliker, CPA* The following table gives the quarterly high and low sales G Completed 9-patient Phase II trial, met primary endpoints Angioedema Anagnostopoulos, Ph.D. EVP Finance and prices of our common stock for the last three years. G Genzyme Corporation joined Dyax as joint venture partner Managing General Partner, Administration and G Complete Phase II 2001 2002 2003 G Gateway Associates, LP Chief Financial Officer Completed 3 of 4 dose cohorts in 48-patient Phase II EDEMA1 trial EDEMA1 study High Low High Low High Low G Susan B. Bayh, J.D. Lynn G. Baird, Ph.D.* Initiated Phase II EDEMA2 trial G Periodically observe James W. Fordyce SVP Development First Quarter $20.94 $6.56 $11.38 $3.10 $2.25 $1.52 G Orphan Drug designation granted in U.S. and Europe effects of repeat dosing in Phase II EDEMA2 Managing Partner, Robert C. Ladner, Ph.D. Second Quarter $19.99 $6.81 $ 4.68 $3.20 $4.90 $1.67 Fordyce & Gabrielson, LLC SVP and Chief DX-88/On-Pump Open Heart Surgery (CABG) study Third Quarter $21.24 $6.05 $ 4.20 $1.65 $7.50 $2.58 Mary Ann Gray, Ph.D. -

Long-Term Follow-Up and Sudden Unexpected Death in Gaucher Disease Type 3 in Egypt
Long-term follow-up and sudden unexpected death in Gaucher disease type 3 in Egypt Magy Abdelwahab, MD, ABSTRACT PhD Objective: To describe the long-term follow-up and distinct phenotype of a large cohort of patients Derek Blankenship, PhD with Gaucher disease type 3 on enzyme replacement therapy (ERT) in Egypt. Raphael Schiffmann, Methods: A prospective cohort study of 78 patients on ERT who were followed for up to 9 years MD, MHSc with yearly evaluations that included EEG and cognitive testing. Results: Of the patients, 73% were homozygous for the L444P GBA1 mutation; all but 7 were Correspondence to neurologically symptomatic. Supranuclear gaze palsy with variable but stable cognitive function Dr. Schiffmann: was present in 91% of patients. Convergent strabismus and bulbar dysfunction were noted in Raphael.schiffmann@ baylorhealth.edu or 22% and 37%, respectively. Features of oppositional defiant disorder were present in 54% of Dr. Abdelwahab: patients. Twenty-three patients (30%) developed seizures while on ERT for 1–9 years. Of those, [email protected] 12 patients (15%) died suddenly and unexpectedly at a mean age of 6.7 6 5.0 years (range 1.5– 18). Sudden death was usually associated with a seizure disorder or a terminal seizure, but 7 of 12 patients had a preceding normal EEG. An additional 11% had background slowing or epilep- togenic activity on EEG without clinical seizures. There were 3 familial cases of sudden unex- pected death. Conclusions: Despite having the most common GBA1 genotype known to be associated with neuronopathic Gaucher disease, patients with Gaucher disease type 3 in Egypt have a phenotype and a clinical outcome on ERT that are very different from those observed in other populations. -

Intravenous Enzyme Replacement Therapy (ERT) for Gaucher Disease
UnitedHealthcare® Oxford Clinical Policy Intravenous Enzyme Replacement Therapy (ERT) for Gaucher Disease Policy Number: PHARMACY 270.16 T2 Effective Date: April 1, 2021 Instructions for Use Table of Contents Page Related Policies Coverage Rationale ....................................................................... 1 • Acquired Rare Disease Drug Therapy Exception Prior Authorization Requirements ................................................ 3 Process Applicable Codes .......................................................................... 3 • Drug Coverage Guidelines Background ................................................................................... 3 • Experimental/Investigational Treatment Benefit Considerations .................................................................. 4 • Experimental/Investigational Treatment for NJ Plans Clinical Evidence ........................................................................... 4 U.S. Food and Drug Administration ............................................. 6 • Provider Administered Drugs – Site of Care References ..................................................................................... 7 Policy History/Revision Information ............................................. 8 Instructions for Use ....................................................................... 8 Coverage Rationale See Benefit Considerations This policy refers to the following drug products, all of which are intravenous enzyme replacement therapies used in the treatment of Gaucher disease: -
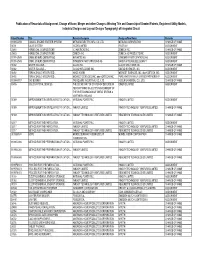
Publication of Recordals of Assignment, Change of Name
Publication of Recordals of Assignment, Change of Name, Merger and other Changes Affecting Title and Ownership of Granted Patents, Registered Utility Models, Industrial Designs and Lay-out Designs Topography) of Integrated Circuit Patent Number Title Patentee/Assignor Assignee/New Name Remarks 1199654059 COAXIAL ENGINE STARTER SYSTEM MITSUBA ELECTRIC MFG. CO. LTD. MITSUBA CORPORATION CHANGE OF NAME 31731 VALVE SYSTEM ISCOR LIMITED IPCOR NV ASSIGNMENT 25483 HERBICIDAL COMPOSITIONS ICI AMERICAS INC. ZENECA INC. CHANGE OF NAME 25483 HERBICIDAL COMPOSITIONS ZENECA INC. ZENECA AG PRODUCTS INC. ASSIGNMENT 1199142549 OXIME ETHERS DERIVATIVES NOVARTIS AG SYNGENTA PARTICIPATIONS AG ASSIGNMENT 1199142549 OXIME ETHERS DERIVATIVES SYNGENTA PARTICIPATIONS AG BAYER AKTIENGESELLSCHAFT ASSIGNMENT 31882 WATER SOLUBLE… GLAXO INC. GLAXO WELLCOME INC. CHANGE OF NAME 31882 WATER SOLUBLE… GLAXO WELLCOME INC. GILEAD SCIENCES, INC. ASSIGNMENT 31492 REPLACEABLE INTEGRATED… AMOS KORIN MIDWEST SCIENCES, INC. dba KORTECH, INC. ASSIGNMENT 31492 REPLACEABLE INTEGRATED… MIDWEST SCIENCES, INC. dba KORTECH, INC. PURE WATER FAMILY LIMITED PARTNERSHIP ASSIGNMENT 1199447817 GAS BURNER TRI-SQUARE INDUSTRIAL CO., LTD. HOSUN UNIVERSAL CO., LTD. CHANGE OF NAME 18495 LIQUID CRYSTAL DEVICES THE SECRETARY OF STATE FOR DEFENSE IN QINETIQ LIMITED ASSIGNMENT HER BRITTANIC MAJESTY'S GOVERNMENT OF THE UNITED KINGDOM OF GREAT BRITAIN & NORTHERN IRELAND 31389 IMPROVEMENTS IN OR RELATING TO CATION… NATIONAL POWER PLC INNOGY LIMITED ASSIGNMENT 31389 IMPROVEMENTS IN OR RELATING TO CATION… -

Estonian Statistics on Medicines 2013 1/44
Estonian Statistics on Medicines 2013 DDD/1000/ ATC code ATC group / INN (rout of admin.) Quantity sold Unit DDD Unit day A ALIMENTARY TRACT AND METABOLISM 146,8152 A01 STOMATOLOGICAL PREPARATIONS 0,0760 A01A STOMATOLOGICAL PREPARATIONS 0,0760 A01AB Antiinfectives and antiseptics for local oral treatment 0,0760 A01AB09 Miconazole(O) 7139,2 g 0,2 g 0,0760 A01AB12 Hexetidine(O) 1541120 ml A01AB81 Neomycin+Benzocaine(C) 23900 pieces A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+Thymol(dental) 2639 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+Cetylpyridinium chloride(gingival) 179340 g A01AD81 Lidocaine+Cetrimide(O) 23565 g A01AD82 Choline salicylate(O) 824240 pieces A01AD83 Lidocaine+Chamomille extract(O) 317140 g A01AD86 Lidocaine+Eugenol(gingival) 1128 g A02 DRUGS FOR ACID RELATED DISORDERS 35,6598 A02A ANTACIDS 0,9596 Combinations and complexes of aluminium, calcium and A02AD 0,9596 magnesium compounds A02AD81 Aluminium hydroxide+Magnesium hydroxide(O) 591680 pieces 10 pieces 0,1261 A02AD81 Aluminium hydroxide+Magnesium hydroxide(O) 1998558 ml 50 ml 0,0852 A02AD82 Aluminium aminoacetate+Magnesium oxide(O) 463540 pieces 10 pieces 0,0988 A02AD83 Calcium carbonate+Magnesium carbonate(O) 3049560 pieces 10 pieces 0,6497 A02AF Antacids with antiflatulents Aluminium hydroxide+Magnesium A02AF80 1000790 ml hydroxide+Simeticone(O) DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 34,7001 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 3,5364 A02BA02 Ranitidine(O) 494352,3 g 0,3 g 3,5106 A02BA02 Ranitidine(P) -

CERDELGA Safely and Effectively
HIGHLIGHTS OF PRESCRIBING INFORMATION ---------------------DOSAGE FORMS AND STRENGTHS---------------------- These highlights do not include all the information needed to use Capsules: 84 mg eliglustat (3) ® CERDELGA safely and effectively. See full prescribing information for ------------------------------CONTRAINDICATIONS------------------------------- CERDELGA. EMs CERDELGA® (eliglustat) capsules, for oral use • Taking a strong or moderate CYP2D6 inhibitor concomitantly with a Initial U.S. Approval: 2014 strong or moderate CYP3A inhibitor. (4, 5.1, 7.1, 12.3) -------------------------RECENT MAJOR CHANGES----------------------------- • Moderate or severe hepatic impairment. (4, 5.3, 8.7, 12.3) Dosage and Administration (2.3) 08/2018 • Mild hepatic impairment taking a strong or moderate CYP2D6 inhibitor. Contraindications (4) 08/2018 (4, 5.3, 8.7, 12.3) Warnings and Precautions (5.1) 08/2018 IMs • --------------------------INDICATIONS AND USAGE---------------------------- Taking a strong or moderate CYP2D6 inhibitor concomitantly with a CERDELGA is a glucosylceramide synthase inhibitor indicated for the long- strong or moderate CYP3A inhibitor. (4, 5.1, 7.1, 12.3) term treatment of adult patients with Gaucher disease type 1 who are CYP2D6 • Taking a strong CYP3A inhibitor. (4, 5.1, 7.1, 12.3) extensive metabolizers (EMs), intermediate metabolizers (IMs), or poor • Any degree of hepatic impairment. (4, 5.3, 8.7, 12.3) metabolizers (PMs) as detected by an FDA-cleared test. (1) PMs • Taking a strong CYP3A inhibitor (4, 5.1, 7.1, 12.3) Limitations of Use: • Any degree of hepatic impairment (4, 5.3, 8.7, 12.3) • CYP2D6 ultra-rapid metabolizers may not achieve adequate concentrations of CERDELGA to achieve a therapeutic effect. (1) ----------------------WARNINGS AND PRECAUTIONS------------------------- • A specific dosage cannot be recommended for CYP2D6 indeterminate ECG Changes and Potential for Cardiac Arrhythmias: Not recommended in metabolizers. -

Lists of Medicinal Products for Rare Diseases in Europe*
March 2021 Lists of medicinal products for rare diseases in Europe* the www.orpha.net www.orphadata.org General Table of contents PART 1: List of orphan medicinal products in Europe with European orphan designation and European marketing authorization 3 Table of contents 3 Methodology 3 Classification by tradename 5 Annex 1: Orphan medicinal products withdrawn from the European Community Register of orphan medicinal products 22 Annex 2: Orphan medicinal products withdrawn from use in the European Union 31 Classification by date of MA in descending order 33 Classification by ATC category 34 Classification by MA holder 35 PART 2 : 37 List of medicinal products intended for rare diseases in Europe with European marketing authorization without an orphan designation in Europe 37 Table of contents 37 Methodology 37 Classification by tradename 38 Classification by date of MA in descending order 104 Classification by ATC category 106 Classification by MA holder 108 For any questions or comments, please contact us: [email protected] Orphanet Report Series - Lists of medicinal products for rare diseases in Europe. March 2021 http://www.orpha.net/orphacom/cahiers/docs/GB/list_of_orphan_drugs_in_europe.pdf 2 PART 1: List of orphan medicinal products in Europe with European orphan designation and European marketing authorization* Table of contents List of orphan medicinal products in Europe with European orphan designation and European marketing authorisation* 3 Methodology 3 Classification by tradename 5 Annex 1: Orphan medicinal products removed or withdrawn from the European Community Register of orphan medicinal products 22 Annex 2: Orphan medicinal products withdrawn from use in the European Union 31 Classification by date of MA in descending order 33 Classification by ATC category 34 Classification by MA holder 35 Methodology This part of the document provides the list of all orphan with the list of medicinal products that have been granted a medicinal products that have received a European Marketing marketing authorization (http://ec.europa.