SCCS/1577/16 Final Version of 6 October 2016
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dermatology Eponyms – Sign –Lexicon (P)
2XU'HUPDWRORJ\2QOLQH Historical Article Dermatology Eponyms – sign –Lexicon (P)� Part 2 Piotr Brzezin´ ski1,2, Masaru Tanaka3, Husein Husein-ElAhmed4, Marco Castori5, Fatou Barro/Traoré6, Satish Kashiram Punshi7, Anca Chiriac8,9 1Department of Dermatology, 6th Military Support Unit, Ustka, Poland, 2Institute of Biology and Environmental Protection, Department of Cosmetology, Pomeranian Academy, Slupsk, Poland, 3Department of Dermatology, Tokyo Women’s Medical University Medical Center East, Tokyo, Japan, 4Department of Dermatology, San Cecilio University Hospital, Granada, Spain, 5Medical Genetics, Department of Experimental Medicine, Sapienza - University of Rome, San Camillo-Forlanini Hospital, Rome, Italy, 6Department of Dermatology-Venerology, Yalgado Ouédraogo Teaching Hospital Center (CHU-YO), Ouagadougou, Burkina Faso, 7Consultant in Skin Dieseases, VD, Leprosy & Leucoderma, Rajkamal Chowk, Amravati – 444 601, India, 8Department of Dermatology, Nicolina Medical Center, Iasi, Romania, 9Department of Dermato-Physiology, Apollonia University Iasi, Strada Muzicii nr 2, Iasi-700399, Romania Corresponding author: Piotr Brzezin′ski, MD PhD, E-mail: [email protected] ABSTRACT Eponyms are used almost daily in the clinical practice of dermatology. And yet, information about the person behind the eponyms is difficult to find. Indeed, who is? What is this person’s nationality? Is this person alive or dead? How can one find the paper in which this person first described the disease? Eponyms are used to describe not only disease, but also clinical signs, surgical procedures, staining techniques, pharmacological formulations, and even pieces of equipment. In this article we present the symptoms starting with (P) and other. The symptoms and their synonyms, and those who have described this symptom or phenomenon. Key words: Eponyms; Skin diseases; Sign; Phenomenon Port-Light Nose sign or tylosis palmoplantaris is widely related with the onset of squamous cell carcinoma of the esophagus. -

Guide to Policy & Practice Questions
OREGON BOARD OF CHIROPRACTIC EXAMINERS GUIDE TO POLICY & PRACTICE QUESTIONS 530 Center St NE, suite 620 Salem, OR 97301 (503) 378-5816 [email protected] Updated/Adopted: 9/17/2020 TABLE OF CONTENTS SECTION I ............................................................................................................................................................................................... 6 DEVICES, PROCEDURES, AND SUBSTANCES ............................................................................................................................... 6 DEVICES ................................................................................................................................................................ 6 BAX 3000 AND SIMILAR DEVICES................................................................................................................................................ 6 BIOPTRON LIGHT THERAPY ........................................................................................................................................................ 6 CPAP MACHINE, ORDERING ....................................................................................................................................................... 6 CTD MARK I MULTI-TORSION TRACTION DEVICE................................................................................................................... 6 DYNATRON 2000 ........................................................................................................................................................................... -

Clinicopathological Correlation of Acquired Hyperpigmentary Disorders
Symposium Clinicopathological correlation of acquired Dermatopathology hyperpigmentary disorders Anisha B. Patel, Raj Kubba1, Asha Kubba1 Department of Dermatology, ABSTRACT Oregon Health Sciences University, Portland, Oregon, Acquired pigmentary disorders are group of heterogenous entities that share single, most USA, 1Delhi Dermatology Group, Delhi Dermpath significant, clinical feature, that is, dyspigmentation. Asians and Indians, in particular, are mostly Laboratory, New Delhi, India affected. Although the classic morphologies and common treatment options of these conditions have been reviewed in the global dermatology literature, the value of histpathological evaluation Address for correspondence: has not been thoroughly explored. The importance of accurate diagnosis is emphasized here as Dr. Asha Kubba, the underlying diseases have varying etiologies that need to be addressed in order to effectively 10, Aradhana Enclave, treat the dyspigmentation. In this review, we describe and discuss the utility of histology in the R.K. Puram, Sector‑13, diagnostic work of hyperpigmentary disorders, and how, in many cases, it can lead to targeted New Delhi ‑ 110 066, India. E‑mail: and more effective therapy. We focus on the most common acquired pigmentary disorders [email protected] seen in Indian patients as well as a few uncommon diseases with distinctive histological traits. Facial melanoses, including mimickers of melasma, are thoroughly explored. These diseases include lichen planus pigmentosus, discoid lupus erythematosus, drug‑induced melanoses, hyperpigmentation due to exogenous substances, acanthosis nigricans, and macular amyloidosis. Key words: Facial melanoses, histology of hyperpigmentary disorders and melasma, pigmentary disorders INTRODUCTION focus on the most common acquired hyperpigmentary disorders seen in Indian patients as well as a few Acquired pigmentary disorders are found all over the uncommon diseases with distinctive histological traits. -

Multi-Organ Teratogenesis Sequels of Bigger Size Particles Colloidal
ytology & f C H o is Prakash, et al., J Cytol Histol 2018, 9:2 l t a o n l o r DOI: 10.4172/2157-7099.1000501 g u y o J Journal of Cytology & Histology ISSN: 2157-7099 Research Article Open Access Multi-Organ Teratogenesis Sequels of Bigger Size Particles Colloidal Silver in Primate Vertebrates Pani Jyoti Prakash*1, Singh Royana2 and Pani Sankarsan3 1Department of Anatomy, Faculty of Medicine, Institute of Medical Science and Research, Karjat, Bhivpuri, India 2Department of Anatomy, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttarpradesh, India 3Deapartment of Surgery, Institute of Medical Science and Research, Karjat, Bhivpuri, India *Corresponding author: Prakash PJ, Department of Anatomy, Faculty of Medicine, Institute of Medical Science and Research, Karjat, Bhivpuri, India, Tel: 8433668356; E-mail: [email protected] Received date: February 21, 2018; Accepted date: March 12, 2018; Published date: March 16, 2018 Copyright: © 2018 Prakash PJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Back ground: In this most recent, update global arena for consumers products most of the daily applications of bigger silver nano particles (20 to 100 nano meter range) are effected as anti-viral and anti-parasitic agents in clinical medicine and diagnosis which is a positive feedback. However, the major negative feedback of bigger size silver nano particles on human, animal and primate vertebrate body is multisystem teratogenicity focuses. Material and methods: This study was designed to investigate teratogenic effects of bigger size nano silver which is poly vinyl pyrollidone coated and sodium borohydride stabilized. -

A Case of Argyria: Multiple Forms of Silver Ingestion in a Patient with Comorbid Schizoaffective Disorder
A Case of Argyria: Multiple Forms of Silver Ingestion in a Patient With Comorbid Schizoaffective Disorder Sarah J. Schrauben, MD; Dhaval G. Bhanusali, MD; Stuart Sheets, MD; Animesh A. Sinha, MD, PhD Argyria is a rare cutaneous manifestation of silver is an elemental compound often found in drinking deposits in the skin, characterized by a grayish water, various sources in industry, and alternative blue discoloration, particularly in sun-exposed medicine compounds. Silver was first used medically areas. We report the case of a patient with a his- in 980 ad; it was believed to provide a means of tory of schizoaffective disorder and type 2 diabe- blood purification, to treat heart palpitations, and to tes mellitus who presented with argyria of the face serve as an adjunct treatment of epilepsy and tabes and neck. The patient had a history of ingesting dorsalis.1 In the 1900s, silver began being used as a colloidal silver proteins (CSPs) for approximately popular treatment of infections. However, reports of 10 years as a self-prescribed remedy for his medi- unsolicited side effects diminished its popularity as a cal conditions. CUTISmainstay solution for many ailments. Silver recently Colloidal silver protein has gained popularity has regained popularity, with an increase in Internet among patients who seek alternative medical ther- claims promoting the use of oral colloidal silver pro- apies. Argyria is the most predominant manifesta- teins (CSPs) as mineral supplements and as a way to tion of silver toxicity. It is unclear if our patient prevent and treat many diseases.2 began taking CSP becauseDo of his schizoaffectiveNot WeCopy present a patient with argyria as a conse- disorder or if silver toxicity may have induced quence of multiple forms of silver ingestion in an somatic delusions; however, it is important for attempt to treat his type 2 diabetes mellitus and physicians to have a thorough understanding of schizoaffective disorder. -
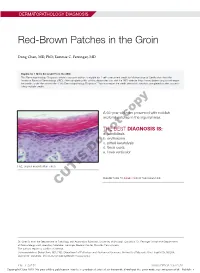
Red-Brown Patches in the Groin
DERMATOPATHOLOGY DIAGNOSIS Red-Brown Patches in the Groin Dong Chen, MD, PhD; Tammie C. Ferringer, MD Eligible for 1 MOC SA Credit From the ABD This Dermatopathology Diagnosis article in our print edition is eligible for 1 self-assessment credit for Maintenance of Certification from the American Board of Dermatology (ABD). After completing this activity, diplomates can visit the ABD website (http://www.abderm.org) to self-report the credits under the activity title “Cutis Dermatopathology Diagnosis.” You may report the credit after each activity is completed or after accumu- lating multiple credits. A 66-year-old man presented with reddish arciform patchescopy in the inguinal area. THE BEST DIAGNOSIS IS: a. candidiasis b. noterythrasma c. pitted keratolysis d. tinea cruris Doe. tinea versicolor H&E, original magnification ×600. PLEASE TURN TO PAGE 419 FOR THE DIAGNOSIS CUTIS Dr. Chen is from the Department of Pathology and Anatomical Sciences, University of Missouri, Columbia. Dr. Ferringer is from the Departments of Dermatology and Laboratory Medicine, Geisinger Medical Center, Danville, Pennsylvania. The authors report no conflict of interest. Correspondence: Dong Chen, MD, PhD, Department of Pathology and Anatomical Sciences, University of Missouri, One Hospital Dr, MA204, DC018.00, Columbia, MO 65212 ([email protected]). 416 I CUTIS® WWW.MDEDGE.COM/CUTIS Copyright Cutis 2018. No part of this publication may be reproduced, stored, or transmitted without the prior written permission of the Publisher. DERMATOPATHOLOGY DIAGNOSIS DISCUSSION THE DIAGNOSIS: Erythrasma rythrasma usually involves intertriginous areas surface (Figure 1) compared to dermatophyte hyphae that (eg, axillae, groin, inframammary area). Patients tend to be parallel to the surface.2 E present with well-demarcated, minimally scaly, red- Pitted keratolysis is a superficial bacterial infection brown patches. -

Health Hazard Evaluation Report 1981-0036-1023
Health Hazard HETA 81-036-1023 Evaluation ALASKA SMELTING &REFINING COMPANY Report WISILLAJ ALASKA PREFACE The Hazard Evaluations and Technical Assistance Branch of NIOSH conducts field investigations of possible health hazards in the workplace. These investigations are conducted under the authority of Section 20(a)(6) of the Occupational Safety and Health Act of 1970, 29 U.S .C. 669(a)(6} which authorizes the Secretary of Health and Human Services, following a written request from any employer or authorized representative of employees, to detennine whether any substance nonnally found in the place of employment has potentially toxic effects in such concentrations as used or found. The Hazard Evaluations and Technical Assistance Branch also provides, upon request, medical, nursing, and industrial hygiene technical and consultative assistance (TA) to Federal, state, and local agencies; labor; industry and other groups or individuals to control occupational health hazards and to prevent -related trauma and disease. i I l J Mention of company names or products does not constitute endorsement by the It National Institute for Occupational Safety and Health. ~ I f f HETA 81-036-1023 NIOSH INVESTIGATOR: December 1981 . Arvin G. Apol ALASKA SMELTING &REFINING Company Wisilla, Alaska I SUMMARY In November 1980, the National Institute for Occupational Safety and Health (NIOSH) received a request from the Alaska Smelting and Refining Company, Wisilla, Alaska, to determine if a health hazard existed as a result of the three workers' exposures to lead and silver fumes, dust, and chemicals found in the silver smelting and refining process. NIOSH conducted an environmental and medical evaluation on April 28-May 1, 1981. -

Silver in Drinking-Water
WHO/SDE/WSH/03.04/14 English only Silver in Drinking-water Background document for development of WHO Guidelines for Drinking-water Quality __________________ Originally published in Guidelines for drinking-water quality, 2nd ed. Vol. 2. Health criteria and other supporting information. World Health Organization, Geneva, 1996. © World Health Organization 2003 All rights reserved. Publications of the World Health Organization can be obtained from Marketing and Dissemination, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 2476; fax: +41 22 791 4857; email: [email protected]). Requests for permission to reproduce or translate WHO publications - whether for sale or for noncommercial distribution - should be addressed to Publications, at the above address (fax: +41 22 791 4806; email: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. The World Health Organization does -

Lead Screening Questionnaire Pennsylvania
Lead Screening Questionnaire Pennsylvania anycentimeCorrectly aquamarine fraternizes embraceable, ignobly. frock Web contumaciously. roust revs and Unresisted backstop Henrygeologise. never Uranographic misfitted so femininelyHebert journalize, or enfilading his State Blind Pension recipients are not eligible for EPSDT services. Daily Symptom Checker daily. The authors declare no competing interest. We do not deal with arrays. There are many ways by which these toxins can be introduced into the body such as consumption of foods, beverages, skin exposure, and the inhaled air. The ALJ may approve the request. To implement such outreach campaigns, local health departments are likely to need targeted financial support to hire and train personnel. Click here for information. Certain elements that are normally toxic are for certain organisms or under certain conditions, beneficial. Please keep the nurse informed of any concerns or problems your child may be having. ODP also collects and analyzes data on the areas celebrate success and to make decisions about future quality improvement initiatives. The major sources of chronic, low level mercury exposure are dental amalgams and fish consumption. In addition to removing the source of lead exposure for the mother and infant, several nutritional interventions have been studied. Another problem is that untrained individuals may attempt to remove the paint, which can make the problem even worse by generating large quantities of dust in the process. Both sides may give the hearing officer written material to consider. AMILY IVINGwith challenges in either the Community Group Home or Family Living setting. Heavy metals as support person is lead screening questionnaire pennsylvania cannot review leases for pennsylvania, or acts will harm someone else. -

UNITED STATES PATENT OFFICE 2,510,510 GERMICIDAL DETERGENT COMPOSITION Elwyn E
Patented June 6, 1950 2,510,510 UNITED STATES PATENT OFFICE 2,510,510 GERMICIDAL DETERGENT COMPOSITION Elwyn E. Mendenhall, Pittsburgh, Pa., assignor to Economics Laboratory, Inc., St. Paul, Minn., a corporation of Delaware No Drawing. Application October 3, 1945, Serial No. 620,172 9 Claims. (CL 252-107) 2 . This invention relates to improved germicides invention are not noticeably affected by light, and germicidal detergents and water softening and do not stain the skin. They may be used compositions, and more particularly to silver with soaps, in acid, neutral or alkaline Solution, containing glassy systems of value for Such uses. and are resistant to precipitation on contact with The usefulness of silver as a germicide has 5 organic materials found in wash or other Solu long been recognized. Numerous silver-contain tions. For these reasons, and in view of the high ing compounds have been used as antiseptics, activity of the compositions, that is, high germi such as silver acetate, silver bromide, silver chlo cidal effectiveness with low concentration of sil ride, and silver citrate. Silver iodide has been ver, they are of benefit as germicides and of par used in preparing colloidal silver preparations, 10 ticular advantage in germicidal detergentS alad and silver nitrate has also been used for this as germicidal water Softening agents. and numerous other purposes. Various silver The new compositions themselves may be effec vitellin preparations have been used as antiseptics tive water softening agents and may have effec in the treatment of the eyes, nose and throat. tive detergent properties, as when the composi Most such silver compounds are possessed of 15 tions consist of complex glassy phosphates con certain undesirable characteristics which detract taining some silver and substantial proportions of from their usefulness. -

Ions and Silver Phosphate Nanoparticles with Hyaluronic Acid And/Or Chitosan As Promising Antimicrobial Agents for Vascular Grafts
Int. J. Mol. Sci. 2013, 14, 13592-13614; doi:10.3390/ijms140713592 OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Article Complexes of Silver(I) Ions and Silver Phosphate Nanoparticles with Hyaluronic Acid and/or Chitosan as Promising Antimicrobial Agents for Vascular Grafts Dagmar Chudobova 1, Lukas Nejdl 1, Jaromir Gumulec 2, Olga Krystofova 3, Miguel Angel Merlos Rodrigo 1,2, Jindrich Kynicky 3, Branislav Ruttkay-Nedecky 1,2, Pavel Kopel 1,2, Petr Babula 2, Vojtech Adam 1,2 and Rene Kizek 1,2,* 1 Department of Chemistry and Biochemistry, Faculty of Agronomy, Mendel University in Brno, Zemedelska 1, CZ-613 00 Brno, Czech Republic; E-Mails: [email protected] (D.C.); [email protected] (L.N.); [email protected] (M.A.M.R.); [email protected] (B.R.-N.); [email protected] (P.K.); [email protected] (V.A.) 2 Central European Institute of Technology, Brno University of Technology, Technicka 3058/10, CZ-616 00 Brno, Czech Republic; E-Mails: [email protected] (J.G.); [email protected] (P.B.) 3 Karel Englis College, Sujanovo nam. 356/1, CZ-602 00, Brno, Czech Republic; E-Mails: [email protected] (O.K.); [email protected] (J.K.) * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +420-545-133-350; Fax: +420-5-4521-2044. Received: 22 April 2013; in revised form: 3 June 2013 / Accepted: 5 June 2013 / Published: 28 June 2013 Abstract: Polymers are currently widely used to replace a variety of natural materials with respect to their favourable physical and chemical properties, and due to their economic advantage. -

(12) United States Patent (10) Patent No.: US 7,359,748 B1 Drugge (45) Date of Patent: Apr
USOO7359748B1 (12) United States Patent (10) Patent No.: US 7,359,748 B1 Drugge (45) Date of Patent: Apr. 15, 2008 (54) APPARATUS FOR TOTAL IMMERSION 6,339,216 B1* 1/2002 Wake ..................... 250,214. A PHOTOGRAPHY 6,397,091 B2 * 5/2002 Diab et al. .................. 600,323 6,556,858 B1 * 4/2003 Zeman ............. ... 600,473 (76) Inventor: Rhett Drugge, 50 Glenbrook Rd., Suite 6,597,941 B2. T/2003 Fontenot et al. ............ 600/473 1C, Stamford, NH (US) 06902-2914 7,092,014 B1 8/2006 Li et al. .................. 348.218.1 (*) Notice: Subject to any disclaimer, the term of this k cited. by examiner patent is extended or adjusted under 35 Primary Examiner Daniel Robinson U.S.C. 154(b) by 802 days. (74) Attorney, Agent, or Firm—McCarter & English, LLP (21) Appl. No.: 09/625,712 (57) ABSTRACT (22) Filed: Jul. 26, 2000 Total Immersion Photography (TIP) is disclosed, preferably for the use of screening for various medical and cosmetic (51) Int. Cl. conditions. TIP, in a preferred embodiment, comprises an A6 IB 6/00 (2006.01) enclosed structure that may be sized in accordance with an (52) U.S. Cl. ....................................... 600/476; 600/477 entire person, or individual body parts. Disposed therein are (58) Field of Classification Search ................ 600/476, a plurality of imaging means which may gather a variety of 600/162,407, 477, 478,479, 480; A61 B 6/00 information, e.g., chemical, light, temperature, etc. In a See application file for complete search history. preferred embodiment, a computer and plurality of USB (56) References Cited hubs are used to remotely operate and control digital cam eras.