Configuration of Monosaccharides
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biochemistry Entry of Fructose and Galactose
Paper : 04 Metabolism of carbohydrates Module : 06 Entry of Fructose and Galactose Dr. Vijaya Khader Dr. MC Varadaraj Principal Investigator Dr.S.K.Khare,Professor IIT Delhi. Paper Coordinator Dr. Ramesh Kothari,Professor UGC-CAS Department of Biosciences Saurashtra University, Rajkot-5, Gujarat-INDIA Dr. S. P. Singh, Professor Content Reviewer UGC-CAS Department of Biosciences Saurashtra University, Rajkot-5, Gujarat-INDIA Dr. Charmy Kothari, Assistant Professor Content Writer Department of Biotechnology Christ College, Affiliated to Saurashtra University, Rajkot-5, Gujarat-INDIA 1 Metabolism of Carbohydrates Biochemistry Entry of Fructose and Galactose Description of Module Subject Name Biochemistry Paper Name 04 Metabolism of Carbohydrates Module Name/Title 06 Entry of Fructose and Galactose 2 Metabolism of Carbohydrates Biochemistry Entry of Fructose and Galactose METABOLISM OF FRUCTOSE Objectives 1. To study the major pathway of fructose metabolism 2. To study specialized pathways of fructose metabolism 3. To study metabolism of galactose 4. To study disorders of galactose metabolism 3 Metabolism of Carbohydrates Biochemistry Entry of Fructose and Galactose Introduction Sucrose disaccharide contains glucose and fructose as monomers. Sucrose can be utilized as a major source of energy. Sucrose includes sugar beets, sugar cane, sorghum, maple sugar pineapple, ripe fruits and honey Corn syrup is recognized as high fructose corn syrup which gives the impression that it is very rich in fructose content but the difference between the fructose content in sucrose and high fructose corn syrup is only 5-10%. HFCS is rich in fructose because the sucrose extracted from the corn syrup is treated with the enzyme that converts some glucose in fructose which makes it more sweet. -
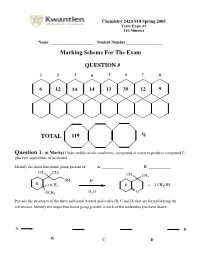
Marking Scheme for the Exam
Chemistry 2420 S10 Spring 2005 Term Exam #1 110 Minutes Name: ______________________ Student Number___________________ Marking Scheme For The Exam QUESTION # 1 2 3 4 5 6 7 8 6 12 14 14 13 39 12 9 TOTAL 119 % Question 1. (6 Marks) Under mildly acidic conditions, compound A reacts to produce compound E plus two equivalents of methanol. Identify the main functional group present in: A: ___________ E: ___________ CH3 CH3 CH 3 CH3 + NH2 H A OCH3 E + 2 CH3OH H O OCH3 2 N Provide the structures of the three additional neutral molecules (B, C and D) that are formed during the conversion. Identify the major functional group present in each of the molecules you have drawn. A E B C D Question 2. (12 Marks) Circle the structure that will best satisfy the given information. - the compound able to undergo an intramolecular cyclization to form a hemiacetal H H HO H HO OH O O O - the compound able to undergo an intramolecular cyclization to form an enamine H H H Me2N MeHN H2N O O O + - the produc t formed from treatment of 2-butanone with NaBD4 followed by a H3O work-up DO H HO D DO D HO H - the compound that would most likely be coloured O H O O - the compound capable of undergoing mutarotation O O O OH OCH3 OH CH3 H CH 3 - the compound that would not react with MeMgBr O O O OH OCH3 OH CH3 H CH3 - the compound bes t described as “anti-aromatic”: - the most stable carbocation + + + Cl Cl Cl OCH 3 H NO2 H H H Question 3. -

REFERENCE ONLY UNIVERSITY of LONDON THESIS This Thesis
REFERENCE ONLY UNIVERSITY OF LONDON THESIS Degree Year Name of Author 0" * COPYRIGHT This is a thesis accepted for a Higher Degree of the University of London. It is an unpublished typescript and the copyright is held by the author. All persons consulting the thesis must read and abide by the Copyright Declaration below. COPYRIGHT DECLARATION I recognise that the copyright of the above-described thesis rests with the author and that no quotation from it or information derived from it may be published without the prior written consent of the author. LOANS Theses may not be lent to individuals, but the Senate House Library may lend a copy to approved libraries within the United Kingdom, for consultation solely on the premises of those libraries. Application should be made to: Inter-Library Loans, Senate House Library, Senate House, Malet Street, London WC1E 7HU. REPRODUCTION University of London theses may not be reproduced without explicit written permission from the Senate House Library. Enquiries should be addressed to the Theses Section of the Library. Regulations concerning reproduction vary according to the date of acceptance of the thesis and are listed below as guidelines. A. Before 1962. Permission granted only upon the prior written consent of the author. (The Senate House Library will provide addresses where possible). B. 1962- 1974. In many cases the author has agreed to permit copying upon completion of a Copyright Declaration. C. 1975 - 1988. Most theses may be copied upon completion of a Copyright Declaration. D. 1989 onwards. Most theses may be copied. This thesis comes within category D. -

Properties and Kinetic Analysis of UDP-Glucose Dehydrogenase from Group a Streptococci IRREVERSIBLE INHIBITION by UDP-CHLOROACETOL*
THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 272, No. 6, Issue of February 7, pp. 3416–3422, 1997 © 1997 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. Properties and Kinetic Analysis of UDP-glucose Dehydrogenase from Group A Streptococci IRREVERSIBLE INHIBITION BY UDP-CHLOROACETOL* (Received for publication, September 19, 1996, and in revised form, November 6, 1996) Robert E. Campbell‡§, Rafael F. Sala‡, Ivo van de Rijn¶, and Martin E. Tanner‡i From the ‡Department of Chemistry, University of British Columbia, Vancouver, British Columbia V6T 1Z1, Canada and ¶Wake Forest University Medical Center, Winston-Salem, North Carolina 27157 UDP-glucuronic acid is used by many pathogenic bac- the capsule enables the bacteria to evade the host’s immune teria in the construction of an antiphagocytic capsule system (7, 8). Group A and C streptococci are mammalian that is required for virulence. The enzyme UDP-glucose pathogens that use UDPGDH in the synthesis of a capsule dehydrogenase catalyzes the NAD1-dependent 2-fold ox- composed of hyaluronic acid (a polysaccharide consisting of idation of UDP-glucose and provides a source of the alternating glucuronic acid and N-acetylglucosamine residues) acid. In the present study the recombinant dehydrogen- (9, 10). Many of the known strains of Streptococcus pneumoniae ase from group A streptococci has been purified and also use UDP-glucuronic acid in the construction of their po- found to be active as a monomer. The enzyme contains lysaccharide capsule (11), and it has recently been shown that no chromophoric cofactors, and its activity is unaffected UDPGDH is required for capsule production in S. -

Part 1 in Our Series of Carbohydrate Lectures. in This Section, You Will Learn About Monosaccharide Structure
Welcome to Part 1 in our series of Carbohydrate lectures. In this section, you will learn about monosaccharide structure. The building blocks of larger carbohydrate polymers. 1 First, let’s review why learning about carbohydrates is important. Carbohydrates are used by biological systems as fuels and energy resources. Carbohydrates typically provide quick energy and are one of the primary energy storage forms in animals. Carbohydrates also provide the precursors to other major macromolecules within the body, including the deoxyribose and ribose required for nucleic acid biosynthesis. Carbohydrates can also provide structural support and cushioning/shock absorption, as well as cell‐cell communication, identification, and signaling. 2 Carbohydrates, as their name implies, are water hydrates of carbon, and they all have the same basic core formula (CH2O)n and are always found in the ratio of 1 carbon to 2 hydrogens to 1 oxygen (1:2:1) making them easy to identify from their molecular formula. 3 Carbohydrates can be divided into subcategories based on their complexity. The simplest carbohydrates are the monosaccharides which are the simple sugars required for the biosynthesis of all the other carbohydrate types. Disaccharides consist of two monosaccharides that have been joined together by a covalent bond called the glycosidic bond. Oligosaccharides are polymers that consist of a few monosaccharides covalently linked together, and Polysaccharides are large polymers that contain hundreds to thousands of monosaccharide units all joined together by glycosidic bonds. The remainder of this lecture will focus on monosaccharides 4 Monosaccharides all have alcohol functional groups associated with them. In addition they also have one additional functional group, either an aldehyde or a ketone. -

Lipids, Carbohydrates, Nucleobases & DNA
Chemistry 2050 “Introduction to Organic Chemistry” Fall Semester 2011 Dr. Rainer Glaser Examination #5: The Final “Lipids, Carbohydrates, Nucleobases & DNA.” Monday, December 12, 2011, 10 am – 12 pm. Name: Answer Key Question 1. Lipids and Detergents. 20 Question 2. Glyceraldehyde and Carbohydrates. 20 Question 3. Nucleobases of DNA and RNA. 20 Question 4. Ribose and Deoxyribose and Their Phosphate Esters. 20 Question 5. Single and Double Strands of DNA. 20 Total 100 — 1 — Question 1. Lipids and Detergents. (20 points) (a) Draw the line segment drawing of glyceryl distearoleate. This fat is the _TRI-ester formed between one molecule of the triol __GLYCEROL_, two molecules of the fatty acid stearic acid, H3C−(CH2)16−COOH, and one molecule of the unsaturated fatty acid oleic acid, H3C−(CH2)7−CH=CH−(CH2)7−COOH. In your drawing, attach the oleic acid to one of the primary alcohols of the triol. Clearly indicate whether the alkene in oleic acid is cis or trans. To convert this fat into soap, one would cook the fat with aqueous NaOH solution, a process that is called __SAPONIFICATION__. (12 points) (b) A simple micelle is shown schematically on the right. Each circle signifies a _________ (polar, nonpolar) head-group, and each wiggly line signifies a ________ (polar, nonpolar) alkyl chain. In the case of “normal” soap, the head- group is a ___CARBOXYLATE__ anion. Indicate where we would find the associated cations (i.e., sodium cations; draw a few in the scheme). Water is on the __________ (inside, outside) of this micelle and fats will accumulate on the _________ (inside, outside) of this micelle. -

Xj 128 IUMP Glucose Substance Will Be Provisionally Referred to As UDPX (Fig
426 Studies on Uridine-Diphosphate-Glucose By A. C. PALADINI AND L. F. LELOIR Instituto de Inve8tigacione&s Bioquimicas, Fundacion Campomar, J. Alvarez 1719, Buenos Aires, Argentina (Received 18 September 1951) A previous paper (Caputto, Leloir, Cardini & found that the substance supposed to be uridine-2'- Paladini, 1950) reported the isolation of the co- phosphate was uridine-5'-phosphate. The hydrolysis enzyme of the galactose -1- phosphate --glucose - 1 - product of UDPG has now been compared with a phosphate transformation, and presented a tenta- synthetic specimen of uridine-5'-phosphate. Both tive structure for the substance. This paper deals substances were found to be identical as judged by with: (a) studies by paper chromatography of puri- chromatographic behaviour (Fig. 1) and by the rate fied preparations of uridine-diphosphate-glucose (UDPG); (b) the identification of uridine-5'-phos- 12A UDPG phate as a product of hydrolysis; (c) studies on the ~~~~~~~~~~~~~(a) alkaline degradation of UDPG, and (d) a substance similar to UDPG which will be referred to as UDPX. UMP Adenosine UDPG preparation8 8tudied by chromatography. 0 UjDPX Paper chromatography with appropriate solvents 0 has shown that some of the purest preparations of UDP UDPG which had been obtained previously contain two other compounds, uridinemonophosphate 0 4 (UMP) and a substance which appears to have the same constitution as UDPG except that it contains an unidentified component instead of glucose. This Xj 128 IUMP Glucose substance will be provisionally referred to as UDPX (Fig. la). The three components have been tested for co- enzymic activity in the galactose-1-phosphate-- 0-4 -J UDPX glucose-l-phosphate transformation, and it has been confirmed that UDPG is the active substance. -

Organic Chemistry by Robert C
(2/94)(6,7,9/95)(8,9/97)(12/99)(1/00) Neuman Chapter 4 Chapter 4 Stereochemistry from Organic Chemistry by Robert C. Neuman, Jr. Professor of Chemistry, emeritus University of California, Riverside [email protected] <http://web.chem.ucsb.edu/~neuman/orgchembyneuman/> Chapter Outline of the Book ************************************************************************************** I. Foundations 1. Organic Molecules and Chemical Bonding 2. Alkanes and Cycloalkanes 3. Haloalkanes, Alcohols, Ethers, and Amines 4. Stereochemistry 5. Organic Spectrometry II. Reactions, Mechanisms, Multiple Bonds 6. Organic Reactions *(Not yet Posted) 7. Reactions of Haloalkanes, Alcohols, and Amines. Nucleophilic Substitution 8. Alkenes and Alkynes 9. Formation of Alkenes and Alkynes. Elimination Reactions 10. Alkenes and Alkynes. Addition Reactions 11. Free Radical Addition and Substitution Reactions III. Conjugation, Electronic Effects, Carbonyl Groups 12. Conjugated and Aromatic Molecules 13. Carbonyl Compounds. Ketones, Aldehydes, and Carboxylic Acids 14. Substituent Effects 15. Carbonyl Compounds. Esters, Amides, and Related Molecules IV. Carbonyl and Pericyclic Reactions and Mechanisms 16. Carbonyl Compounds. Addition and Substitution Reactions 17. Oxidation and Reduction Reactions 18. Reactions of Enolate Ions and Enols 19. Cyclization and Pericyclic Reactions *(Not yet Posted) V. Bioorganic Compounds 20. Carbohydrates 21. Lipids 22. Peptides, Proteins, and α−Amino Acids 23. Nucleic Acids ************************************************************************************** -

Ii- Carbohydrates of Biological Importance
Carbohydrates of Biological Importance 9 II- CARBOHYDRATES OF BIOLOGICAL IMPORTANCE ILOs: By the end of the course, the student should be able to: 1. Define carbohydrates and list their classification. 2. Recognize the structure and functions of monosaccharides. 3. Identify the various chemical and physical properties that distinguish monosaccharides. 4. List the important monosaccharides and their derivatives and point out their importance. 5. List the important disaccharides, recognize their structure and mention their importance. 6. Define glycosides and mention biologically important examples. 7. State examples of homopolysaccharides and describe their structure and functions. 8. Classify glycosaminoglycans, mention their constituents and their biological importance. 9. Define proteoglycans and point out their functions. 10. Differentiate between glycoproteins and proteoglycans. CONTENTS: I. Chemical Nature of Carbohydrates II. Biomedical importance of Carbohydrates III. Monosaccharides - Classification - Forms of Isomerism of monosaccharides. - Importance of monosaccharides. - Monosaccharides derivatives. IV. Disaccharides - Reducing disaccharides. - Non- Reducing disaccharides V. Oligosaccarides. VI. Polysaccarides - Homopolysaccharides - Heteropolysaccharides - Carbohydrates of Biological Importance 10 CARBOHYDRATES OF BIOLOGICAL IMPORTANCE Chemical Nature of Carbohydrates Carbohydrates are polyhydroxyalcohols with an aldehyde or keto group. They are represented with general formulae Cn(H2O)n and hence called hydrates of carbons. -
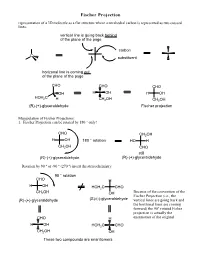
Fischer Projection Representation of a 3D Molecule As a Flat Structure Where a Tetrahedral Carbon Is Represented As Two Crossed Lines
Fischer Projection representation of a 3D molecule as a flat structure where a tetrahedral carbon is represented as two crossed lines. vertical line is going back behind of the plane of the page carbon C substituent horizonal line is coming out of the plane of the page CHO CHO CHO OH H OH H OH HOH2C H CH2OH CH2OH (R)-(+)-glyceraldehyde Fischer projection Manipulation of Fischer Projections: 1. Fischer Projection can be rotated by 180 ° only! CHO CH2OH H OH 180 ° rotation HO H CH2OH CHO still (R)-(+)-glyceraldehyde (R)-(+)-glyceraldehyde Rotation by 90 ° or -90 ° (270 °) invert the stereochemistry 90 ° rotation CHO H H OH HOH2C CHO CH2OH OH Because of the convention of the Fischer Projection (i.e., the (R)-(+)-glyceraldehyde (S)-(-)-glyceraldehyde vertical lines are going back and the horizonal lines are coming forward) the 90° rotated Fisher projection is actually the CHO H enantiomer of the original H OH HOH2C CHO CH2OH OH These two compounds are enantiomers 2. If one group of the Fischer projection is held steady, the other groups can be rotated either clockwise or counter clockwise. hold this group steady CHO CHO H OH HOH2C H CH2OH OH still (R)-(+)-glyceraldehyde (R)-(+)-glyceraldehyde Assigning R and S-configurations to Fischer projections 1. Assign priorities to the four substituents according to the Cahn-Ingold-Prelog convention. 2. Perform the two allowed manipulations of the Fischer projection to place the lowest priority group on one of the vertical positions (either top or bottom) 3. If the priorities of the other three groups (1-2-3) proceed clockwise, the stereogenic center is assigned as R. -

The Effects of Glucose, N-Acetylglucosamine, Glyceraldehyde and Other Sugars on Insulin Release in Vivo S
Diabetologia 11,279-284 (1975) by Springer-Verlag 1975 The Effects of Glucose, N-Acetylglucosamine, Glyceraldehyde and Other Sugars on Insulin Release in Vivo S. J.H. Ashcroft and J.R. Crossley Department of Biochemistry, University of Bristol, England Received: February 18, 1975, and in revised form: April 28, 1975 Summary. The specificity for carbohydrates of insulin secretory duced hyperglycaemia, but peak insulin concentrations occurred responses in vivo was studied. Test sugars were injected via a left before any change in plasma glucose concentration. No evidence femoral vein cannula into conscious rats. Blood samples collected was obtained for a stimulatory effect of galactose on insulinrelease. over the ensuing 60 min via a left femoral arterial cannula were Infusion for 60 rain of N-acetylglucosarnine produced a sustained assayed for plasma insulin and glucose, and, in some experiments, elevated plasma insulin concentration and significant hypogly- for N-acetyl glucosamine. Whereas L-glucose or saline produced no caemia. The present in vivo results agree with previous in vitro significant changes in plasma insulin or glucose concentrations, observations, and could indicate a role for sugars other than glucose D-glucose, N-acetylglucosamine, D-glucosamine, fructose, D-glyc- in the regulation of insulin release. eraldehyde and DL-glyceraldehyde were potent secretagogues. Simultaneous injection of mannoheptulose abolished the insulin- Key words: N-acetylglucosamine, insulin release, glyceraldehy- otropic action of glucose and N-acetylglucosamine, but not of DL- de, glucose, glucosamine, fructose, galactose, mannoheptulose, glu- glyceraldehyde. Fructose, glucosamine, and DL-glyceraldehyde in- coreceptor. Detailed studies of the specificity of the insulin se- been conclusively established. Strong evidence for the cretory response to sugars have been performed with involvement of a metabolite has been provided by the mouse and rat islets of Langerhans in vitro [1, 2]. -

UDP-Galactose 4′-Epimerase from the Liver fluke, Fasciola Hepatica: Biochemical Characterization of the Enzyme and Identification of Inhibitors
463 UDP-galactose 4′-epimerase from the liver fluke, Fasciola hepatica: biochemical characterization of the enzyme and identification of inhibitors VERONIKA L. ZINSSER1, STEFFEN LINDERT2, SAMANTHA BANFORD1, ELIZABETH M. HOEY1, ALAN TRUDGETT1,3 and DAVID J. TIMSON1,3* 1 School of Biological Sciences, Queen’s University Belfast, Medical Biology Centre, 97 Lisburn Road, Belfast BT9 7BL, UK 2 Department of Pharmacology, Center for Theoretical Biological Physics, University of California San Diego, La Jolla, CA 92093, USA 3 Institute for Global Food Security, Queen’s University Belfast, 18-30 Malone Road, Belfast BT9 5BN, UK (Received 12 June 2014; accepted 19 July 2014; first published online 15 August 2014) SUMMARY The Leloir pathway enzyme uridine diphosphate (UDP)-galactose 4′-epimerase from the common liver fluke Fasciola hepatica (FhGALE) was identified and characterized. The enzyme can be expressed in, and purified from, Escherichia coli. The recombinant enzyme is active: the Km (470 μM) is higher than the corresponding human enzyme (HsGALE), whereas −1 + the kcat (2·3 s ) is substantially lower. FhGALE binds NAD and has shown to be dimeric by analytical gel filtration. Like the human and yeast GALEs, FhGALE is stabilized by the substrate UDP-galactose. Molecular modelling predicted that FhGALE adopts a similar overall fold to HsGALE and that tyrosine 155 is likely to be the catalytically critical residue in the active site. In silico screening of the National Cancer Institute Developmental Therapeutics Program library identified 40 potential inhibitors of FhGALE which were tested in vitro. Of these, 6 showed concentration-dependent inhibition of FhGALE, some with nanomolar IC50 values.