Hermann Emil Fischer: Life and Achievements
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Peter P. T. Sah and the Synthesis of Vitamin C in China and Europe
EASTM 20 (2003 ): 92-98 Peter P. T. Sah and the Synthesis of Vitamin C in China and Europe Zhang Li [Zhang Li is Associate Professor at the Institute for the History of Natural Sci ence, Chinese Academy of Sciences. She has published a number of articles on the history of modern chemistry of both the West and China and the social his tory of science in twentieth-century China, including studies on the influence of higher education reform on chemical education in the 1950s in China (1992) and the coordination between national needs and scientists' autonomy during 1949-/965 (2003). She recently received her doctoral degree in the Philosophy of Science from Peking University, completing a dissertation on the institution alization of science in the People's Republic of China. Her forthcoming book is called Gaofenzi kexue zai Zhongguo de jianli ( 1949-1965) r%'J 5t r f-4 ~ ft i:p 00 R"J ~ JI. (1949-1965) ( Institutionalization of Polymer Science in China(l949- /965) Jinan: Shandong jiaoyu chubanshe 2003, ¥ff 1¥i : W J'.f: ¥!I.. W tB It& ffr±, 2003).J * * * The synthesis of vitamin C was one of the main scientific achievements in the 1930s. Many scientists in Europe made contributions to this field, especially Albert Szent-Gyorgyi (1893-1986) from Hungary and Sir Walter Norman Ha worth ( 1883-1950) from England, both of whom won the Nobel Prize in 1937. In the same period, a Chinese chemist, Sa Bentie ~ :;$: ~ (1900-1986), better known outside China as Peter P. T. Sah, was also studying vitamin C. -

Curriculum Vitae Markus Fischer Date of Birth 20 June 1962
Curriculum vitae Markus Fischer Date of birth 20 June 1962 Nationality German and Swiss Affiliation Institute of Plant Sciences, University of Bern Altenbergrain 21, 3013 Bern, Switzerland Phone +41 31 631 4943, Secretary 4911, Fax 4942 E-mail [email protected] Fields of Expertise Drivers and ecosystem-service consequences of biodiversity change Ecology and evolution of rare and invasive species Conservation biology, mountain ecology Work at the science-society and science-policy interfaces Employment Since 2007 Full Professor of Plant Ecology, Institute of Plant Sciences, University of Bern and (since 2010) Director of the Botanical Garden of the University of Bern Since 2012 Director, Institute of Plant Sciences, University of Bern Since 2012 Guest researcher, Senckenberg Biodiversity and Climate Institute, Frankfurt, Germany 2008-2011 Head, Biology Department, University of Bern 2007-2012 Guest Professor at the University of Potsdam, Germany 2003-2007 Full Professor of Botany and Community Ecology, Institute of Biochemistry and Biology, and Director of the Botanical Garden, University of Potsdam 1996-2003 Main Assistant, Institute of Environmental Sciences, Univ. of Zurich, Switzerland 1993-1996 Research Associate, Botanical Institute, University of Basel, Switzerland 1990 Research Associate, Department of Mechanical Engineering, University of Maryland, College Park, U.S.A. Education 1982-1989 Studies and MSc Diploma in Physics, Technical University Munich, Germany 1990–1992 Studies and BSc in Biology, University of Basel, -

CV Otto Diels
Curriculum Vitae Prof. Dr. Otto P. H. Diels Name: Otto Paul Hermann Diels Lebensdaten: 23. Januar 1876 – 7. März 1954 Otto Diels war ein deutscher Chemiker. Für die auch nach ihm benannte Diels‐Alder‐Reaktion (auch Diensynthese genannt) erhielt er 1950 gemeinsam mit seinem Schüler Kurt Alder den Nobelpreis für Chemie. Gemeinsam mit Emil Abderhalden arbeitete er über Gallensteine und begann, sich mit Cholesterin zu beschäftigen. Darüber hinaus trägt die Diels‐Säure seinen Namen, eine von ihm bei der Strukturaufklärung der Steroide synthetisierte Dicarbonsäure. Akademischer und beruflicher Werdegang Otto Diels studierte ab 1895 an der Universität Berlin die Fächer Chemie, Physik, Botanik, Mineralogie und Philosophie. Er wurde im Sommer 1899 beim späteren Nobelpreisträger für Chemie, Emil Fischer, mit einer Arbeit über Cyanur‐Verbindungen promoviert. Im selben Jahr wurde er Assistent am Chemischen Institut der Universität Berlin. 1904 folgte die Habilitation. 1913 wurde er im selben Institut Abteilungsvorsteher. 1914 wurde er an der Universität Berlin zum außerordentlichen Professor ernannt. Zwei Jahre später nahm er einen Ruf nach Kiel an. Dort wurde er Professor für Chemie und Direktor des Chemischen Instituts und fungierte im akademischen Jahr 1925/26 als Rektor der Kieler Universität. Er blieb seiner Alma mater bis an sein Lebensende treu, trotz mehrfacher Berufungen an andere Hochschulen, darunter nach Gießen und Berlin. Nobelpreis für Chemie 1950 Bereits 1911 begann Diels, den Azodicarbonsäurediethylester zu untersuchen. Diese kurz „Azoester“ genannte Verbindung führte ihn schließlich zu seinem wichtigsten Arbeitsgebiet, der Untersuchung der Dien‐Synthese, wie die Reaktion eines Diens mit einem reaktiven Alken oder Alkin zunächst genannt wurde. Einzelne Experimente, die Diels schließlich als Dien‐Synthesen erkannte, waren bereits kurz nach der Jahrhundertwende veröffentlicht worden. -

Goverdhan Mehta Chemistry - a 21St Century Science for Global Sustainability: Is It Future Ready?
Goverdhan Mehta Chemistry - A 21st Century Science for Global Sustainability: Is it future ready? Goverdhan Mehta A ‘selfie’ with theSchool chemical of Chemistry world…… University of Hyderabad National Geophysical Research Institute, CSIR Foundation Day, Sept. 27, 2019 Introducing Chemistry through the Lens of Earth's Systems: What Role Can Systems Thinking Play in Developing Chemically and Environmentally Literate Citizens? J. Kornfeld, S. Stokoe. J. Chemical Education 2019, 96, 2910-2917 A bouquet of ‘matters’ that matter New symbols Passion Sustainability Legacies Ethics & values Responsible Connections Systems Directions Humility Ideas & icons Inspirations Un mélange de beaucoup de choses “Chemistry ought not to be for chemists alone” - Miguel de Unamuno ‘…Life, Universe and Everything’ Chemistry – a source of happiness…. Chem -Connectome ‘...I feel sorry for people who don’t know anything about chemistry. They are missing an important source of happiness....’ - Linus Pauling 1901-1994 S.A. Matlin, G. Mehta, H. Hopf. Chemistry Embraced by All. Science 2015, 347, 1179 Chemistry is in everything. and everything is in it, it is the basis of life, without it we wouldn't exist. Green tea has ~ 200 chemicals Coffee has ~ 1000 chemicals Wine has >1000 chemicals Light cigarette ~ 4000 chemicals Chemistry is ubiquitous/omnipresent Chemistry – Tracing the roots and to the present BCE Art & craft of mixing substances A giant knowledge leap Alchemy to modern science Evidence based science Discipline in a Table - systematization Mendeleev’s Periodic Law ‘Molecularization’ of chemical matter 20th Century A century of evolutionary march of chemistry 20h Century Value added products from almost anything “Utility science” and everything Molecular understanding of life processes and “Core Science” chemical matter Interdisciplinarity in forefront “Integrative Science” Resource stressed planet “Sustainability Science” 21st Century S. -

The Lock-And-Key Analogy in 20Th Century Biochemistry
From: Rebecca Mertens The Construction of Analogy-Based Research Programs The Lock-and-Key Analogy in 20th Century Biochemistry April 2019, 224 p., pb., ill. 34,99 € (DE), 978-3-8376-4442-5 E-Book: PDF: 34,99 € (DE), ISBN 978-3-8394-4442-9 When the German chemist Emil Fischer presented his lock-and-key hypothesis in 1899, his analogy to describe the molecular relationship between enzymes and substrates quickly gained vast influence and provided future generations of scientists with a tool to investigate the relation between chemical structure and biological specificity. Rebecca Mertens explains the appeal of the lock-and-key analogy by its role in model building and in the construction of long-term, cross-generational research programs. She argues that a crucial feature of these research programs, namely ascertaining the continuity of core ideas and concepts, is provided by a certain way of analogy-based modelling. Rebecca Mertens (PhD), born in 1984, is a postdoctoral researcher in the history and philosophy of science at the University of Bielefeld, Germany. She works on the role of analogies, models and forms of comparison in the history of molecular genetics and is a member of the collaborative research program "Practices of ComparisonÚ Ordering and Changing the World". During her graduate and doctoral studies, she was a visiting scholar at the École Normale Supérieure in Paris and a visiting graduate fellow at the Minnesota Center for Philosophy of Science. For further information: www.transcript-verlag.de/en/978-3-8376-4442-5 © 2019 -

Curriculum Vitae Prof. Dr. Adolf Otto Reinhold Windaus
Curriculum Vitae Prof. Dr. Adolf Otto Reinhold Windaus Name: Adolf Otto Reinhold Windaus Lebensdaten: 25. Dezember 1876 - 9. Juni 1956 Adolf Windaus war ein deutscher Chemiker. Er untersuchte Naturstoffe, vor allem die biochemisch wichtigen Sterine und ihren Zusammenhang mit anderen Naturstoffen. Er entdeckte die chemische Verwandtschaft von Cholesterin und Gallensäure. Außerdem lieferte er Arbeiten über Vitamine, vor allem das Vitamin D. Zwischen 1927 und 1931 gelang ihm die Isolierung mehrerer D-Vitamine. Seine Forschungen bildeten die Grundlage für später von seinen Schülern durchgeführten Arbeiten über die menschlichen Sexualhormone. Für seine Verdienste um die Erforschung des Aufbaus der Sterine und ihres Zusammenhangs mit den Vitaminen wurde Adolf Windaus 1928 mit dem Nobelpreis für Chemie ausgezeichnet. Akademischer und beruflicher Werdegang Adolf Windaus begann 1895 ein Studium der Medizin an der Universität Freiburg. Er wechselte nach Berlin, wo er 1897 das Physikum bestand. 1899 wurde er in Freiburg mit einer Arbeit über Neue Beiträge zur Kenntnis der Digitalisstoffe promoviert wurde. 1901 war er zunächst in Berlin als Assistent von Emil Fischer (Nobelpreis für Chemie 1902) tätig. Während dieser Zeit wandte er sich zunehmend chemischen Fragestellungen zu. Außerdem begann er mit seinen Forschungen zu den Sterinen. 1903 habilitierte er sich in Freiburg mit einer Arbeit über Cholesterin. 1906 erhielt er eine außerordentliche Professur an der Universität Göttingen. Im Anschluss wechselte er für zwei Jahre an die Universität Innsbruck, wo er eine außerordentliche Professur für angewandte medizinische Chemie erhielt. 1915 ging er zurück nach Göttingen, wo er als Nachfolger von Otto Wallach (Nobelpreis für Chemie 1910) Ordinarius für Chemie wurde. Dort blieb er bis zu seiner Emeritierung im Jahr 1944. -

Peptide Chemistry up to Its Present State
Appendix In this Appendix biographical sketches are compiled of many scientists who have made notable contributions to the development of peptide chemistry up to its present state. We have tried to consider names mainly connected with important events during the earlier periods of peptide history, but could not include all authors mentioned in the text of this book. This is particularly true for the more recent decades when the number of peptide chemists and biologists increased to such an extent that their enumeration would have gone beyond the scope of this Appendix. 250 Appendix Plate 8. Emil Abderhalden (1877-1950), Photo Plate 9. S. Akabori Leopoldina, Halle J Plate 10. Ernst Bayer Plate 11. Karel Blaha (1926-1988) Appendix 251 Plate 12. Max Brenner Plate 13. Hans Brockmann (1903-1988) Plate 14. Victor Bruckner (1900- 1980) Plate 15. Pehr V. Edman (1916- 1977) 252 Appendix Plate 16. Lyman C. Craig (1906-1974) Plate 17. Vittorio Erspamer Plate 18. Joseph S. Fruton, Biochemist and Historian Appendix 253 Plate 19. Rolf Geiger (1923-1988) Plate 20. Wolfgang Konig Plate 21. Dorothy Hodgkins Plate. 22. Franz Hofmeister (1850-1922), (Fischer, biograph. Lexikon) 254 Appendix Plate 23. The picture shows the late Professor 1.E. Jorpes (r.j and Professor V. Mutt during their favorite pastime in the archipelago on the Baltic near Stockholm Plate 24. Ephraim Katchalski (Katzir) Plate 25. Abraham Patchornik Appendix 255 Plate 26. P.G. Katsoyannis Plate 27. George W. Kenner (1922-1978) Plate 28. Edger Lederer (1908- 1988) Plate 29. Hennann Leuchs (1879-1945) 256 Appendix Plate 30. Choh Hao Li (1913-1987) Plate 31. -
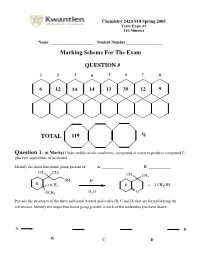
Marking Scheme for the Exam
Chemistry 2420 S10 Spring 2005 Term Exam #1 110 Minutes Name: ______________________ Student Number___________________ Marking Scheme For The Exam QUESTION # 1 2 3 4 5 6 7 8 6 12 14 14 13 39 12 9 TOTAL 119 % Question 1. (6 Marks) Under mildly acidic conditions, compound A reacts to produce compound E plus two equivalents of methanol. Identify the main functional group present in: A: ___________ E: ___________ CH3 CH3 CH 3 CH3 + NH2 H A OCH3 E + 2 CH3OH H O OCH3 2 N Provide the structures of the three additional neutral molecules (B, C and D) that are formed during the conversion. Identify the major functional group present in each of the molecules you have drawn. A E B C D Question 2. (12 Marks) Circle the structure that will best satisfy the given information. - the compound able to undergo an intramolecular cyclization to form a hemiacetal H H HO H HO OH O O O - the compound able to undergo an intramolecular cyclization to form an enamine H H H Me2N MeHN H2N O O O + - the produc t formed from treatment of 2-butanone with NaBD4 followed by a H3O work-up DO H HO D DO D HO H - the compound that would most likely be coloured O H O O - the compound capable of undergoing mutarotation O O O OH OCH3 OH CH3 H CH 3 - the compound that would not react with MeMgBr O O O OH OCH3 OH CH3 H CH3 - the compound bes t described as “anti-aromatic”: - the most stable carbocation + + + Cl Cl Cl OCH 3 H NO2 H H H Question 3. -

Named Organic Reactions (U – Z)
Dr. John Andraos, http://www.careerchem.com/NAMED/Named-Rxns(U-Z).pdf 1 NAMED ORGANIC REACTIONS (U – Z) © Dr. John Andraos, 2000 - 2014 Department of Chemistry, York University 4700 Keele Street, Toronto, ONTARIO M3J 1P3, CANADA For suggestions, corrections, additional information, and comments please send e-mails to [email protected] http://www.chem.yorku.ca/NAMED/ Sakae Uemura Japanese, b. Japan Uemura oxidation Nishimura, T.; Onoue, T.; Ohe, K.; Uemura S. Tetrahedron Lett. 1998 , 39 , 6011 Nishimura, T.; Onoue, T.; Ohe, K.; Uemura S. J. Org. Chem. 1999 , 64 , 6750 Biographical references: Ivar Karl Ugi 5 September 1930 – 29 September 2005 German, b. Kuressaare, Estland, Germany Ugi condensation Ugi, I.; Meyr, R.; Fetzer, U.; Steinbrueckner, C. Angew. Chem. 1959 , 71 , 386 Urban, R.; Ugi, I., Angew. Chem. Int. Engl. Ed . 1975 , 14 , 61 Biographical references: Chemical Research Faculties: An International Directory 1996 , American Chemical Society: Washington, D.C., 1996 Wer ist Wer? Das Deutsche Who’s Who 1999/2000 , Schmidt-Römhild: Lübeck, 1999 Lemmen, P.; Fontain, E.; Bauer, J. Angew. Chem. Int. Ed. 2006 , 45 , 193 Fritz Ullmann 2 July 1875 - 17 March 1939 German, b. Fürth, Germany Ullmann reaction Ullmann, F. Ann. Chem . 1904 , 332 , 38 Dr. John Andraos, http://www.careerchem.com/NAMED/Named-Rxns(U-Z).pdf 2 Biographical references: Meyer, K.H., Helv. Chim. Acta 1940 , 23 , 93 Upjohn reaction Van Rheenen, V.; Kelly, R.C.; Cha, D.Y. Tetrahedron Lett. 1976 , 1973 A. Verley French, b. ? Meerwein-Pondorff-Verley reduction see Hans Leberecht Meerwein Biographical references: Victor Villiger 1 September 1868 - 1934 Swiss, b. -

Otto Hahn Otto Hahn
R.N. 70269/98 Postal Registration No.: DL-SW-1/4082/15-17 ISSN : 0972-169X Date of posting: 26-27 of advance month Date of publication: 24 of advance month May 2017 Vol. 19 No. 8 Rs. 5.00 Otto Hahn Discoverer of Nuclear Fission Editorial: Consolidating 35 science communication activities in our country Otto Hahn: Discoverer of 34 Nuclear Fission Keep Your Eyes Healthy 31 Phenol: A Serious 30 Environmental Threat Accidental Discoveries in 28 Medical Science Cures for haemorrhoids— 24 Simple treatments and Surgeries Recent developments 21 in science and technology 36 Editorial Consolidating science communication activities in our country Dr. R. Gopichandran It is well known that the National Council of Science Museums of India’s leadership in science technology and innovation (STI) across the Ministry of Culture, Government of India, the National Institute the bilateral and multilateral framework also. The news feature service of Science Communication and Information Resources (NISCAIR) and the portal activity have well defined action plans to reach out to of CSIR, the National Council for Science and Technology fellow institutions and citizens with suitably embellished platform Communication (NCSTC) of the Department of Science and and opportunities for all to deliver together. Technology (DST), Government of India and Vigyan Prasar, also While these are interesting and extremely important, especially of DST, have been carrying out excellent science communication because they respond to the call to upscale and value add science activities over the years. It cannot be denied that the reach has been and technology communication, it is equally important to document quite significant collectively. -

Antibodies with Some Bite Antibodies Have Yet to Be Determined, It Is Reasonable to Suppose That the Ester Or David E
-~-------------------------------------N8NSANDVIEWS----------------_N_A_TU_R_E_V-O_L_._32_5_22_J_A_N_U_A_RY__ 19~87 Enzyme catalysis kinetics and is subject to competitive inhibition. Although the detailed chemical mechanisms of these catalytic Antibodies with some bite antibodies have yet to be determined, it is reasonable to suppose that the ester or David E. Hansen carbamate is strained towards a tetra hedral geometry on binding, thus facilitat THE spectacular specificities and rate William Jencks', more directly, stated in ing the attack of the hydroxide ion. Fur enhancements of enzymes are without 1969 thermore, the Scripps group has already equal among all known catalysts. It is no If complementarity between active site and found that their antibody can act via both wonder then that the design of new en transition state contributes significantly to nucleophilic and general base catalysis, zymes has long been a goal of biochemists. enzyme catalysis, it should be possible to syn depending on the substrate used. Now two independent groups, one led by thesize an enzyme by constructing a binding The fact that this approach succeeded at Alfonso Tramontano and Richard Lerner site. One way to do this is to prepare an anti all gives great hope to those attempting to of the Scripps Clinic and Research Foun body to a haptenic group which resembles the isolate even more efficient antibody cat dation', and the other by Peter Schultz of transition state of a given reaction. The com alysts by this and by other strategies. In the University of California at Berkeley\ bining sites of such antibodies should be com addition, it may be possible to modify have taken steps towards this goal by plementary to the transition state and should cause an acceleration by forcing bound sub existing catalytic antibodies. -

Robert Burns Woodward
The Life and Achievements of Robert Burns Woodward Long Literature Seminar July 13, 2009 Erika A. Crane “The structure known, but not yet accessible by synthesis, is to the chemist what the unclimbed mountain, the uncharted sea, the untilled field, the unreached planet, are to other men. The achievement of the objective in itself cannot but thrill all chemists, who even before they know the details of the journey can apprehend from their own experience the joys and elations, the disappointments and false hopes, the obstacles overcome, the frustrations subdued, which they experienced who traversed a road to the goal. The unique challenge which chemical synthesis provides for the creative imagination and the skilled hand ensures that it will endure as long as men write books, paint pictures, and fashion things which are beautiful, or practical, or both.” “Art and Science in the Synthesis of Organic Compounds: Retrospect and Prospect,” in Pointers and Pathways in Research (Bombay:CIBA of India, 1963). Robert Burns Woodward • Graduated from MIT with his Ph.D. in chemistry at the age of 20 Woodward taught by example and captivated • A tenured professor at Harvard by the age of 29 the young... “Woodward largely taught principles and values. He showed us by • Published 196 papers before his death at age example and precept that if anything is worth 62 doing, it should be done intelligently, intensely • Received 24 honorary degrees and passionately.” • Received 26 medals & awards including the -Daniel Kemp National Medal of Science in 1964, the Nobel Prize in 1965, and he was one of the first recipients of the Arthur C.