Part 1 in Our Series of Carbohydrate Lectures. in This Section, You Will Learn About Monosaccharide Structure
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fructose and Sucrose Intake Increase Exogenous Carbohydrate Oxidation During Exercise
nutrients Article Fructose and Sucrose Intake Increase Exogenous Carbohydrate Oxidation during Exercise Jorn Trommelen 1, Cas J. Fuchs 1, Milou Beelen 1, Kaatje Lenaerts 1, Asker E. Jeukendrup 2, Naomi M. Cermak 1 and Luc J. C. van Loon 1,* 1 NUTRIM School of Nutrition and Translational Research in Metabolism, Maastricht University Medical Centre, P.O. Box 616, 6200 MD Maastricht, The Netherlands; [email protected] (J.T.); [email protected] (C.J.F.); [email protected] (M.B.); [email protected] (K.L.); [email protected] (N.M.C.) 2 School of Sport, Exercise and Health Sciences, Loughborough University, Loughborough LE11 3TU, UK; [email protected] * Correspondence: [email protected]; Tel.: +31-43-388-1397 Received: 6 January 2017; Accepted: 16 February 2017; Published: 20 February 2017 Abstract: Peak exogenous carbohydrate oxidation rates typically reach ~1 g·min−1 during exercise when ample glucose or glucose polymers are ingested. Fructose co-ingestion has been shown to further increase exogenous carbohydrate oxidation rates. The purpose of this study was to assess the impact of fructose co-ingestion provided either as a monosaccharide or as part of the disaccharide sucrose on exogenous carbohydrate oxidation rates during prolonged exercise in trained cyclists. −1 −1 Ten trained male cyclists (VO2peak: 65 ± 2 mL·kg ·min ) cycled on four different occasions for −1 180 min at 50% Wmax during which they consumed a carbohydrate solution providing 1.8 g·min of glucose (GLU), 1.2 g·min−1 glucose + 0.6 g·min−1 fructose (GLU + FRU), 0.6 g·min−1 glucose + 1.2 g·min−1 sucrose (GLU + SUC), or water (WAT). -

Pentose PO4 Pathway, Fructose, Galactose Metabolism.Pptx
Pentose PO4 pathway, Fructose, galactose metabolism The Entner Doudoroff pathway begins with hexokinase producing Glucose 6 PO4 , but produce only one ATP. This pathway prevalent in anaerobes such as Pseudomonas, they doe not have a Phosphofructokinase. The pentose phosphate pathway (also called the phosphogluconate pathway and the hexose monophosphate shunt) is a biochemical pathway parallel to glycolysis that generates NADPH and pentoses. While it does involve oxidation of glucose, its primary role is anabolic rather than catabolic. There are two distinct phases in the pathway. The first is the oxidative phase, in which NADPH is generated, and the second is the non-oxidative synthesis of 5-carbon sugars. For most organisms, the pentose phosphate pathway takes place in the cytosol. For each mole of glucose 6 PO4 metabolized to ribulose 5 PO4, 2 moles of NADPH are produced. 6-Phosphogluconate dh is not only an oxidation step but it’s also a decarboxylation reaction. The primary results of the pathway are: The generation of reducing equivalents, in the form of NADPH, used in reductive biosynthesis reactions within cells (e.g. fatty acid synthesis). Production of ribose-5-phosphate (R5P), used in the synthesis of nucleotides and nucleic acids. Production of erythrose-4-phosphate (E4P), used in the synthesis of aromatic amino acids. Transketolase and transaldolase reactions are similar in that they transfer between carbon chains, transketolases 2 carbon units or transaldolases 3 carbon units. Regulation; Glucose-6-phosphate dehydrogenase is the rate- controlling enzyme of this pathway. It is allosterically stimulated by NADP+. The ratio of NADPH:NADP+ is normally about 100:1 in liver cytosol. -

Carbohydrate Food List
Carbohydrate Food List 1. Breads, grains, and pasta Portion Size Carbs (g) Bread 1 slice 10-20 Cornbread 1 piece (deck of 30 cards) Cornmeal (Dry) 2 Tbsp 12 Cream of wheat, cooked with water ½ cup 15 Croutons ½ cup 12 Flour, all-purpose, dry 2 Tbsp 12 Oatmeal, cooked with water ½ cup 12-15 Pasta, cooked 1 cup 45 Pita bread 6” to 9” pita 30-45 Rice, cooked 1 cup 45 Tortilla corn 6” tortilla 12 Tortilla flour 6” tortilla 15 2. Nuts and Legumes Portion size Carbs (g) Beans (black, pinto, refried) and ½ cup 18-22 lentils, as prepared Hummus ½ cup 15-20 Nuts, mixed ½ cup 15 3. Starchy Vegetables Portion size Carbs (g) Corn on the cob 6” to 9” ear 20-30 Corn, cooked or canned ½ cup 15 Peas ½ cup 12 Potato, baked 1 medium (6 oz) 40 Adult Diabetes Education Program - 1 - Potato, mashed ½ cup 15-20 Sweet potato/yams 1 medium (5 oz) 25 Winter squash (butternut, acorn, 1 cup 15-30 hubbard), cooked 4. Milk and yogurts Portion size Carbs (g) Almond milk (plain, unsweetened) 1 cup <1 Cow’s milk (fat-free, 1%, 2%, whole) 1 cup 12 Soy milk (plain, unsweetened) 1 cup 3 Yogurt (plain) 1 cup 14 Yogurt, Greek (plain) 1 cup 10 5. Fruits Portion Size Carbs (g) Apple 1 medium 15-30 (tennis ball) Applesauce (unsweetened) ½ cup 15 Apricots, dried 7 pieces 15 Banana 6”-9” 30-45 Blackberries, blueberries 1 cup 20 Cherries 12 15 Dates, dried 5-6 dates 30 Fruit cocktail, canned (in own juice) ½ cup 15 Grapefruit ½ large 15 Grapes 15 15 Kiwi 1 small (egg) 15 Mango, cubed and frozen ½ cup 15 Melons, cantaloupe or honeydew 1 cup 15 Orange 1 medium 15 (tennis ball) Adult Diabetes Education Program Carbohydrates Food List - 2 - Peaches, canned (in own juice) ½ cup 15 Pear 6 oz 20 Pineapple (fresh) 1 cup, diced 20 Plum 1 plum 10 Prunes, dried 3 prunes 15 Raisins 2 Tbsp 15 Raspberries 1 cup 15 Strawberries 1 cup halves 12 Watermelon 1 cup diced 12 6. -

Carbohydrate Counting
Carbohydrate Counting What is Carbohydrate Counting? Carbohydrate counting is a meal-planning tool that many people use to manage their blood sugar. Carbohydrate counting, or ‘carb counting’, is done by tracking the amount of carbohydrates eaten at meals and snacks. Carbohydrates are a type of nutrient found in starchy and sweet foods. When starchy or sweet foods are eaten, the body breaks the carbohydrates in the foods down into glucose. This glucose is released into the blood. Another name for glucose in the blood is blood sugar. There are four steps in carbohydrate Foods with carbohydrates: counting: • Soda pop, juice, Tang, Kool-Aid, and other sweet drinks • Starchy vegetables like potatoes, corn, squash, beans and peas 1. Timing • Rice, noodles, oatmeal, cereals, breads, crackers 2. Amount • Milk and yogurt 3. Balance • Beans 4. Monitoring • Chips, cookies, cakes, ice cream Step 1: Timing • Fruit and fruit juice A moderate amount of carbohydrate foods gets broken down into a moderate In diabetes meal planning, one choice of carbohydrates has about 15 grams of amount of blood sugar. carbohydrates. For most people trying to keep their blood sugars in a healthy range, eating about 3 or 4 choices of carbohydrate foods (45-60 grams) at a meal is a good place to start. A good amount of carbohydrates for snacks is usually around 1-2 carb + = Blood sugar 140 choices (or 15-30 grams). Food List of Carbohydrate Choices Two hours after eating one sandwich, this person’s blood sugar is 140. Each serving has about 15 grams of Carbohydrates Choose 3-4 choices (or 45-60 grams) of these foods per meal Choose 1-2 choices (or 15-30 grams) of these foods per snack + = Blood sugar 210 Starches Bread or large pilot bread 1 piece Cooked rice or noodles 1/3 cup Oatmeal, cooked 1/2 cup Two hours after eating two sandwiches, the same person’s blood sugar is 210. -
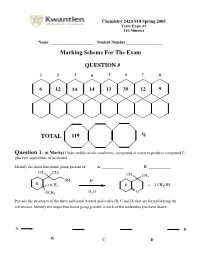
Marking Scheme for the Exam
Chemistry 2420 S10 Spring 2005 Term Exam #1 110 Minutes Name: ______________________ Student Number___________________ Marking Scheme For The Exam QUESTION # 1 2 3 4 5 6 7 8 6 12 14 14 13 39 12 9 TOTAL 119 % Question 1. (6 Marks) Under mildly acidic conditions, compound A reacts to produce compound E plus two equivalents of methanol. Identify the main functional group present in: A: ___________ E: ___________ CH3 CH3 CH 3 CH3 + NH2 H A OCH3 E + 2 CH3OH H O OCH3 2 N Provide the structures of the three additional neutral molecules (B, C and D) that are formed during the conversion. Identify the major functional group present in each of the molecules you have drawn. A E B C D Question 2. (12 Marks) Circle the structure that will best satisfy the given information. - the compound able to undergo an intramolecular cyclization to form a hemiacetal H H HO H HO OH O O O - the compound able to undergo an intramolecular cyclization to form an enamine H H H Me2N MeHN H2N O O O + - the produc t formed from treatment of 2-butanone with NaBD4 followed by a H3O work-up DO H HO D DO D HO H - the compound that would most likely be coloured O H O O - the compound capable of undergoing mutarotation O O O OH OCH3 OH CH3 H CH 3 - the compound that would not react with MeMgBr O O O OH OCH3 OH CH3 H CH3 - the compound bes t described as “anti-aromatic”: - the most stable carbocation + + + Cl Cl Cl OCH 3 H NO2 H H H Question 3. -

Carbohydrates: Structure and Function
CARBOHYDRATES: STRUCTURE AND FUNCTION Color index: . Very important . Extra Information. “ STOP SAYING I WISH, START SAYING I WILL” 435 Biochemistry Team *هذا العمل ﻻ يغني عن المصدر المذاكرة الرئيسي • The structure of carbohydrates of physiological significance. • The main role of carbohydrates in providing and storing of energy. • The structure and function of glycosaminoglycans. OBJECTIVES: 435 Biochemistry Team extra information that might help you 1-synovial fluid: - It is a viscous, non-Newtonian fluid found in the cavities of synovial joints. - the principal role of synovial fluid is to reduce friction between the articular cartilage of synovial joints during movement O 2- aldehyde = terminal carbonyl group (RCHO) R H 3- ketone = carbonyl group within (inside) the compound (RCOR’) 435 Biochemistry Team the most abundant organic molecules in nature (CH2O)n Carbohydrates Formula *hydrate of carbon* Function 1-provides important part of energy Diseases caused by disorders of in diet . 2-Acts as the storage form of energy carbohydrate metabolism in the body 3-structural component of cell membrane. 1-Diabetesmellitus. 2-Galactosemia. 3-Glycogen storage disease. 4-Lactoseintolerance. 435 Biochemistry Team Classification of carbohydrates monosaccharides disaccharides oligosaccharides polysaccharides simple sugar Two monosaccharides 3-10 sugar units units more than 10 sugar units Joining of 2 monosaccharides No. of carbon atoms Type of carbonyl by O-glycosidic bond: they contain group they contain - Maltose (α-1, 4)= glucose + glucose -Sucrose (α-1,2)= glucose + fructose - Lactose (β-1,4)= glucose+ galactose Homopolysaccharides Heteropolysaccharides Ketone or aldehyde Homo= same type of sugars Hetero= different types Ketose aldose of sugars branched unBranched -Example: - Contains: - Contains: Examples: aldehyde group glycosaminoglycans ketone group. -

1. Nucleotides A. Pentose Sugars – 5-Carbon Sugar 1) Deoxyribose – in DNA 2) Ribose – in RNA B. Phosphate Group C. Nitroge
1. Nucleotides a. Pentose sugars – 5-Carbon sugar 1) Deoxyribose – in DNA 2) Ribose – in RNA b. Phosphate group c. Nitrogenous bases 1) Purines a) Adenine b) Guanine 2) Pyrimidines a) Cytosine b) Thymine 2. Types of Nucleic Acids a. DNA 1) Locations 2) Functions b. RNA 1) Locations 2) Functions E. High Energy Biomolecules 1. Adenosine triphosphate a. Uses 1) Active transport 2) Movement 3) Biosynthesis reactions b. Regeneration 1) ADP + Pi + Energy → ATP 4. Classes of proteins a. Structural – ex. Collagen, keratin b. Transport – Hemoglobin, many β-globulins c. Contractile – Actin and Myosin of muscle tissue d. Regulatory - Hormones e. Immunologic - Antibodies f. Clotting – Thrombin and Fibrin g. Osmotic - Albumin h. Catalytic – Enzymes 1) Characteristics of enzymes • Proteins (most); ribonucleoproteins (few/ribozymes) • Act as organic catalysts • Lower the activation energy of reactions • Not changed by the reaction • Bind to their substrates o Lock-and-key model of enzyme activity o Induced-fit model • Highly specific • Named by adding -ase to substrate name; e.g., maltose/maltase • May require cofactors which may be: o Nonprotein metal ions such as copper, manganese, potassium, sodium o Small organic molecules known as coenzymes. The B vitamins like thiamine (B1) riboflavin (B2) and nicotinamide are precursors of coenzymes. • May require activation; e.g., pepsinogen pepsin in stomach chief cells 4. Factors Affecting Enzyme Action • pH o pepsin (stomach) @ pH = 2; trypsin (small int.) @ pH = 8 • Temperature o Denatured by high temp’s. • Enzyme inhibitors o Competitive inhibitors o Noncompetitive inhibitors • Effect of substrate concentration and reversible reactions and the Law of Mass D. -

PENTOSE PHOSPHATE PATHWAY — Restricted for Students Enrolled in MCB102, UC Berkeley, Spring 2008 ONLY
Metabolism Lecture 5 — PENTOSE PHOSPHATE PATHWAY — Restricted for students enrolled in MCB102, UC Berkeley, Spring 2008 ONLY Bryan Krantz: University of California, Berkeley MCB 102, Spring 2008, Metabolism Lecture 5 Reading: Ch. 14 of Principles of Biochemistry, “Glycolysis, Gluconeogenesis, & Pentose Phosphate Pathway.” PENTOSE PHOSPHATE PATHWAY This pathway produces ribose from glucose, and it also generates 2 NADPH. Two Phases: [1] Oxidative Phase & [2] Non-oxidative Phase + + Glucose 6-Phosphate + 2 NADP + H2O Ribose 5-Phosphate + 2 NADPH + CO2 + 2H ● What are pentoses? Why do we need them? ◦ DNA & RNA ◦ Cofactors in enzymes ● Where do we get them? Diet and from glucose (and other sugars) via the Pentose Phosphate Pathway. ● Is the Pentose Phosphate Pathway just about making ribose sugars from glucose? (1) Important for biosynthetic pathways using NADPH, and (2) a high cytosolic reducing potential from NADPH is sometimes required to advert oxidative damage by radicals, e.g., ● - ● O2 and H—O Metabolism Lecture 5 — PENTOSE PHOSPHATE PATHWAY — Restricted for students enrolled in MCB102, UC Berkeley, Spring 2008 ONLY Two Phases of the Pentose Pathway Metabolism Lecture 5 — PENTOSE PHOSPHATE PATHWAY — Restricted for students enrolled in MCB102, UC Berkeley, Spring 2008 ONLY NADPH vs. NADH Metabolism Lecture 5 — PENTOSE PHOSPHATE PATHWAY — Restricted for students enrolled in MCB102, UC Berkeley, Spring 2008 ONLY Oxidative Phase: Glucose-6-P Ribose-5-P Glucose 6-phosphate dehydrogenase. First enzymatic step in oxidative phase, converting NADP+ to NADPH. Glucose 6-phosphate + NADP+ 6-Phosphoglucono-δ-lactone + NADPH + H+ Mechanism. Oxidation reaction of C1 position. Hydride transfer to the NADP+, forming a lactone, which is an intra-molecular ester. -

REFERENCE ONLY UNIVERSITY of LONDON THESIS This Thesis
REFERENCE ONLY UNIVERSITY OF LONDON THESIS Degree Year Name of Author 0" * COPYRIGHT This is a thesis accepted for a Higher Degree of the University of London. It is an unpublished typescript and the copyright is held by the author. All persons consulting the thesis must read and abide by the Copyright Declaration below. COPYRIGHT DECLARATION I recognise that the copyright of the above-described thesis rests with the author and that no quotation from it or information derived from it may be published without the prior written consent of the author. LOANS Theses may not be lent to individuals, but the Senate House Library may lend a copy to approved libraries within the United Kingdom, for consultation solely on the premises of those libraries. Application should be made to: Inter-Library Loans, Senate House Library, Senate House, Malet Street, London WC1E 7HU. REPRODUCTION University of London theses may not be reproduced without explicit written permission from the Senate House Library. Enquiries should be addressed to the Theses Section of the Library. Regulations concerning reproduction vary according to the date of acceptance of the thesis and are listed below as guidelines. A. Before 1962. Permission granted only upon the prior written consent of the author. (The Senate House Library will provide addresses where possible). B. 1962- 1974. In many cases the author has agreed to permit copying upon completion of a Copyright Declaration. C. 1975 - 1988. Most theses may be copied upon completion of a Copyright Declaration. D. 1989 onwards. Most theses may be copied. This thesis comes within category D. -

Carbohydrates Adapted from Pellar
Carbohydrates Adapted from Pellar OBJECTIVE: To learn about carbohydrates and their reactivities BACKGROUND: Carbohydrates are a major food source, with most dietary guidelines recommending that 45-65% of daily calories come from carbohydrates. Rice, potatoes, bread, pasta and candy are all high in carbohydrates, specifically starches and sugars. These compounds are just a few examples of carbohydrates. Other carbohydrates include fibers such as cellulose and pectins. In addition to serving as the primary source of energy for the body, sugars play a number of other key roles in biological processes, such as forming part of the backbone of DNA structure, affecting cell-to-cell communication, nerve and brain cell function, and some disease pathways. Carbohydrates are defined as polyhydroxy aldehydes or polyhydroxy ketones, or compounds that break down into these substances. They can be categorized according to the number of carbons in the structure and whether a ketone or an aldehyde group is present. Glucose, for example, is an aldohexose because it contains six carbons and an aldehyde functional group. Similarly, fructose would be classified as a ketohexose. glucose fructose A more general classification scheme exists where carbohydrates are broken down into the groups monosaccharides, disaccharides, and polysaccharides. Monosaccharides are often referred to as simple sugars. These compounds cannot be broken down into smaller sugars by acid hydrolysis. Glucose, fructose and ribose are examples of monosaccharides. Monosaccharides exist mostly as cyclic structures containing hemiacetal or hemiketal groups. These structures in solution are in equilibrium with the corresponding open-chain structures bearing aldehyde or ketone functional groups. The chemical linkage of two monosaccharides forms disaccharides. -

Multi-Enzymatic Cascades in the Synthesis of Modified Nucleosides
biomolecules Article Multi-Enzymatic Cascades in the Synthesis of Modified Nucleosides: Comparison of the Thermophilic and Mesophilic Pathways Ilja V. Fateev , Maria A. Kostromina, Yuliya A. Abramchik, Barbara Z. Eletskaya , Olga O. Mikheeva, Dmitry D. Lukoshin, Evgeniy A. Zayats , Maria Ya. Berzina, Elena V. Dorofeeva, Alexander S. Paramonov , Alexey L. Kayushin, Irina D. Konstantinova * and Roman S. Esipov Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry RAS, Miklukho-Maklaya 16/10, 117997 GSP, B-437 Moscow, Russia; [email protected] (I.V.F.); [email protected] (M.A.K.); [email protected] (Y.A.A.); [email protected] (B.Z.E.); [email protected] (O.O.M.); [email protected] (D.D.L.); [email protected] (E.A.Z.); [email protected] (M.Y.B.); [email protected] (E.V.D.); [email protected] (A.S.P.); [email protected] (A.L.K.); [email protected] (R.S.E.) * Correspondence: [email protected]; Tel.: +7-905-791-1719 ! Abstract: A comparative study of the possibilities of using ribokinase phosphopentomutase ! nucleoside phosphorylase cascades in the synthesis of modified nucleosides was carried out. Citation: Fateev, I.V.; Kostromina, Recombinant phosphopentomutase from Thermus thermophilus HB27 was obtained for the first time: M.A.; Abramchik, Y.A.; Eletskaya, a strain producing a soluble form of the enzyme was created, and a method for its isolation and B.Z.; Mikheeva, O.O.; Lukoshin, D.D.; chromatographic purification was developed. It was shown that cascade syntheses of modified nu- Zayats, E.A.; Berzina, M.Y..; cleosides can be carried out both by the mesophilic and thermophilic routes from D-pentoses: ribose, Dorofeeva, E.V.; Paramonov, A.S.; 2-deoxyribose, arabinose, xylose, and 2-deoxy-2-fluoroarabinose. -

DNA Stands for Deoxyribose Nucleic Acid
DNA and Protein Synthesis DNA • DNA stands for deoxyribose nucleic acid. • This chemical substance is found in the nucleus of all cells in all living organisms • DNA controls all the chemical changes which take place in cells • The kind of cell which is formed, (muscle, blood, nerve etc) is controlled by DNA Ribose is a sugar, like glucose, but with only five carbon atoms in its molecule. Deoxyribose is almost the same but lacks one oxygen atom. The nitrogen bases are: o ADENINE (A) o THYMINE (T) o CYTOSINE (C) o GUANINE (G) Nucleotides • A molecule of DNA is formed by millions of nucleotides joined together in a long chain. • DNA is a very large molecule made up of a long chain of sub-units. • The sub-units are called nucleotides. • Each nucleotide is made up of a sugar called deoxyribose, a phosphate group -PO4 and an organic (Nitrogen) base: A, T, C, G BASE PAIRING RULE amount of C= amount of G AND amount of A= amount of T • Adenine always pairs with thymine, and guanine always pairs with cytosine. DNA STRUCTURE • The nucleotide bases will point to the inside of the DNA molecule while the outside (backbone) of the DNA molecule will be made of the sugar and phosphate molecules. • When complete the DNA molecule forms a double helix (two spiral sides wrapped together). • The paired strands are coiled into a spiral called A DOUBLE HELIX. Genes • Each chromosome contains hundreds of genes. • Most of your characteristics: hair color, height, how things taste to a person, are determined by the kinds of proteins cells make (gene).