Organic Chemistry by Robert C
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prebiological Evolution and the Metabolic Origins of Life
Prebiological Evolution and the Andrew J. Pratt* Metabolic Origins of Life University of Canterbury Keywords Abiogenesis, origin of life, metabolism, hydrothermal, iron Abstract The chemoton model of cells posits three subsystems: metabolism, compartmentalization, and information. A specific model for the prebiological evolution of a reproducing system with rudimentary versions of these three interdependent subsystems is presented. This is based on the initial emergence and reproduction of autocatalytic networks in hydrothermal microcompartments containing iron sulfide. The driving force for life was catalysis of the dissipation of the intrinsic redox gradient of the planet. The codependence of life on iron and phosphate provides chemical constraints on the ordering of prebiological evolution. The initial protometabolism was based on positive feedback loops associated with in situ carbon fixation in which the initial protometabolites modified the catalytic capacity and mobility of metal-based catalysts, especially iron-sulfur centers. A number of selection mechanisms, including catalytic efficiency and specificity, hydrolytic stability, and selective solubilization, are proposed as key determinants for autocatalytic reproduction exploited in protometabolic evolution. This evolutionary process led from autocatalytic networks within preexisting compartments to discrete, reproducing, mobile vesicular protocells with the capacity to use soluble sugar phosphates and hence the opportunity to develop nucleic acids. Fidelity of information transfer in the reproduction of these increasingly complex autocatalytic networks is a key selection pressure in prebiological evolution that eventually leads to the selection of nucleic acids as a digital information subsystem and hence the emergence of fully functional chemotons capable of Darwinian evolution. 1 Introduction: Chemoton Subsystems and Evolutionary Pathways Living cells are autocatalytic entities that harness redox energy via the selective catalysis of biochemical transformations. -

Chapter 3 • Ratio: Cnh2n+2
Organic Chemistry, 5th Edition Alkane Formulas L. G. Wade, Jr. • All C-C single bonds • Saturated with hydrogens Chapter 3 • Ratio: CnH2n+2 Structure and Stereochemistry • Alkane homologs: CH3(CH2)nCH3 of Alkanes • Same ratio for branched alkanes H H H H H H CH H C C C C H H H H C CCH Jo Blackburn H H H H H H Richland College, Dallas, TX H => Butane, C4H10 Dallas County Community College District Chapter 3Isobutane, C H 2 © 2003, Prentice Hall 4 10 Common Names Pentanes H H H H H H H CH • Isobutane, “isomer of butane” H C C C C C H H H • Isopentane, isohexane, etc., methyl H H H H H H H C CCC H branch on next-to-last carbon in chain. H H H H • Neopentane, most highly branched n-pentane, C5H12 isopentane, C5H12 • Five possible isomers of hexane, CH3 18 isomers of octane and 75 for H3C C CH3 decane! CH3 => => neopentane, C5H12 Chapter 3 3 Chapter 3 4 Longest Chain IUPAC Names • The number of carbons in the longest • Find the longest continuous carbon chain determines the base name: chain. ethane, hexane. (Listed in Table 3.2, • Number the carbons, starting closest to page 81.) the first branch. • If there are two possible chains with the • Name the groups attached to the chain, same number of carbons, use the chain using the carbon number as the locator. with the most substituents. • Alphabetize substituents. H3C CH CH CH • Use di-, tri-, etc., for multiples of same 2 3 CH3 substituent. -

Stereochemistry of Alkanes and Cycloalkanes
STEREOCHEMISTRY OF ALKANES AND CYCLOALKANES CONFORMATIONAL ISOMERS 1 CONFORMATIONAL ISOMERS • Stereochemistry concerned with the 3-D aspects of molecules • Rotation is possible around C-C bonds in open- chain molecules • A conformation is one of the many possible arrangements of atoms caused by rotation about a single bond, and a specific conformation is called a conformer (conformational isomer). 2 SOME VOCABULARY • A staggered conformation is the conformation in which all groups on two adjacent carbons are as far from each other as possible. • An eclipsed conformation is the conformation in which all groups on two adjacent carbons are as close to each other as possible. • Dihedral angle is angle between the substituents on two adjacent carbons; changes with rotation around the C-C bond 3 MORE VOCABULARY • Angle strain is the strain caused by the deformation of a bond angle from its normal value. • Steric strain is the repulsive interaction caused by atoms attempting to occupy the same space. • Torsional strain is the repulsive interaction between two bonds as they rotate past each other. Torsional strain is responsible for the barrier to rotation around the C-C bond. The eclipsed from higher in energy than the staggered form. 4 CONFORMATION OF ETHANE • Conformation- Different arrangement of atoms resulting from bond rotation • Conformations can be represented in 2 ways: 5 TORSIONAL STRAIN • We do not observe perfectly free rotation • There is a barrier to rotation, and some conformers are more stable than others • Staggered- most stable: -
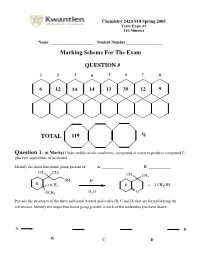
Marking Scheme for the Exam
Chemistry 2420 S10 Spring 2005 Term Exam #1 110 Minutes Name: ______________________ Student Number___________________ Marking Scheme For The Exam QUESTION # 1 2 3 4 5 6 7 8 6 12 14 14 13 39 12 9 TOTAL 119 % Question 1. (6 Marks) Under mildly acidic conditions, compound A reacts to produce compound E plus two equivalents of methanol. Identify the main functional group present in: A: ___________ E: ___________ CH3 CH3 CH 3 CH3 + NH2 H A OCH3 E + 2 CH3OH H O OCH3 2 N Provide the structures of the three additional neutral molecules (B, C and D) that are formed during the conversion. Identify the major functional group present in each of the molecules you have drawn. A E B C D Question 2. (12 Marks) Circle the structure that will best satisfy the given information. - the compound able to undergo an intramolecular cyclization to form a hemiacetal H H HO H HO OH O O O - the compound able to undergo an intramolecular cyclization to form an enamine H H H Me2N MeHN H2N O O O + - the produc t formed from treatment of 2-butanone with NaBD4 followed by a H3O work-up DO H HO D DO D HO H - the compound that would most likely be coloured O H O O - the compound capable of undergoing mutarotation O O O OH OCH3 OH CH3 H CH 3 - the compound that would not react with MeMgBr O O O OH OCH3 OH CH3 H CH3 - the compound bes t described as “anti-aromatic”: - the most stable carbocation + + + Cl Cl Cl OCH 3 H NO2 H H H Question 3. -

Chapter 4: Stereochemistry Introduction to Stereochemistry
Chapter 4: Stereochemistry Introduction To Stereochemistry Consider two of the compounds we produced while finding all the isomers of C7H16: CH3 CH3 2-methylhexane 3-methylhexane Me Me Me C Me H Bu Bu Me Me 2-methylhexane H H mirror Me rotate Bu Me H 2-methylhexame is superimposable with its mirror image Introduction To Stereochemistry Consider two of the compounds we produced while finding all the isomers of C7H16: CH3 CH3 2-methylhexane 3-methylhexane H C Et Et Me Pr Pr 3-methylhexane Me Me H H mirror Et rotate H Me Pr 2-methylhexame is superimposable with its mirror image Introduction To Stereochemistry Consider two of the compounds we produced while finding all the isomers of C7H16: CH3 CH3 2-methylhexane 3-methylhexane .Compounds that are not superimposable with their mirror image are called chiral (in Greek, chiral means "handed") 3-methylhexane is a chiral molecule. .Compounds that are superimposable with their mirror image are called achiral. 2-methylhexane is an achiral molecule. .An atom (usually carbon) with 4 different substituents is called a stereogenic center or stereocenter. Enantiomers Et Et Pr Pr Me CH3 Me H H 3-methylhexane mirror enantiomers Et Et Pr Pr Me Me Me H H Me H H Two compounds that are non-superimposable mirror images (the two "hands") are called enantiomers. Introduction To Stereochemistry Structural (constitutional) Isomers - Compounds of the same molecular formula with different connectivity (structure, constitution) 2-methylpentane 3-methylpentane Conformational Isomers - Compounds of the same structure that differ in rotation around one or more single bonds Me Me H H H Me H H H H Me H Configurational Isomers or Stereoisomers - Compounds of the same structure that differ in one or more aspects of stereochemistry (how groups are oriented in space - enantiomers or diastereomers) We need a a way to describe the stereochemistry! Me H H Me 3-methylhexane 3-methylhexane The CIP System Revisited 1. -

REFERENCE ONLY UNIVERSITY of LONDON THESIS This Thesis
REFERENCE ONLY UNIVERSITY OF LONDON THESIS Degree Year Name of Author 0" * COPYRIGHT This is a thesis accepted for a Higher Degree of the University of London. It is an unpublished typescript and the copyright is held by the author. All persons consulting the thesis must read and abide by the Copyright Declaration below. COPYRIGHT DECLARATION I recognise that the copyright of the above-described thesis rests with the author and that no quotation from it or information derived from it may be published without the prior written consent of the author. LOANS Theses may not be lent to individuals, but the Senate House Library may lend a copy to approved libraries within the United Kingdom, for consultation solely on the premises of those libraries. Application should be made to: Inter-Library Loans, Senate House Library, Senate House, Malet Street, London WC1E 7HU. REPRODUCTION University of London theses may not be reproduced without explicit written permission from the Senate House Library. Enquiries should be addressed to the Theses Section of the Library. Regulations concerning reproduction vary according to the date of acceptance of the thesis and are listed below as guidelines. A. Before 1962. Permission granted only upon the prior written consent of the author. (The Senate House Library will provide addresses where possible). B. 1962- 1974. In many cases the author has agreed to permit copying upon completion of a Copyright Declaration. C. 1975 - 1988. Most theses may be copied upon completion of a Copyright Declaration. D. 1989 onwards. Most theses may be copied. This thesis comes within category D. -

Part 1 in Our Series of Carbohydrate Lectures. in This Section, You Will Learn About Monosaccharide Structure
Welcome to Part 1 in our series of Carbohydrate lectures. In this section, you will learn about monosaccharide structure. The building blocks of larger carbohydrate polymers. 1 First, let’s review why learning about carbohydrates is important. Carbohydrates are used by biological systems as fuels and energy resources. Carbohydrates typically provide quick energy and are one of the primary energy storage forms in animals. Carbohydrates also provide the precursors to other major macromolecules within the body, including the deoxyribose and ribose required for nucleic acid biosynthesis. Carbohydrates can also provide structural support and cushioning/shock absorption, as well as cell‐cell communication, identification, and signaling. 2 Carbohydrates, as their name implies, are water hydrates of carbon, and they all have the same basic core formula (CH2O)n and are always found in the ratio of 1 carbon to 2 hydrogens to 1 oxygen (1:2:1) making them easy to identify from their molecular formula. 3 Carbohydrates can be divided into subcategories based on their complexity. The simplest carbohydrates are the monosaccharides which are the simple sugars required for the biosynthesis of all the other carbohydrate types. Disaccharides consist of two monosaccharides that have been joined together by a covalent bond called the glycosidic bond. Oligosaccharides are polymers that consist of a few monosaccharides covalently linked together, and Polysaccharides are large polymers that contain hundreds to thousands of monosaccharide units all joined together by glycosidic bonds. The remainder of this lecture will focus on monosaccharides 4 Monosaccharides all have alcohol functional groups associated with them. In addition they also have one additional functional group, either an aldehyde or a ketone. -

Lipids, Carbohydrates, Nucleobases & DNA
Chemistry 2050 “Introduction to Organic Chemistry” Fall Semester 2011 Dr. Rainer Glaser Examination #5: The Final “Lipids, Carbohydrates, Nucleobases & DNA.” Monday, December 12, 2011, 10 am – 12 pm. Name: Answer Key Question 1. Lipids and Detergents. 20 Question 2. Glyceraldehyde and Carbohydrates. 20 Question 3. Nucleobases of DNA and RNA. 20 Question 4. Ribose and Deoxyribose and Their Phosphate Esters. 20 Question 5. Single and Double Strands of DNA. 20 Total 100 — 1 — Question 1. Lipids and Detergents. (20 points) (a) Draw the line segment drawing of glyceryl distearoleate. This fat is the _TRI-ester formed between one molecule of the triol __GLYCEROL_, two molecules of the fatty acid stearic acid, H3C−(CH2)16−COOH, and one molecule of the unsaturated fatty acid oleic acid, H3C−(CH2)7−CH=CH−(CH2)7−COOH. In your drawing, attach the oleic acid to one of the primary alcohols of the triol. Clearly indicate whether the alkene in oleic acid is cis or trans. To convert this fat into soap, one would cook the fat with aqueous NaOH solution, a process that is called __SAPONIFICATION__. (12 points) (b) A simple micelle is shown schematically on the right. Each circle signifies a _________ (polar, nonpolar) head-group, and each wiggly line signifies a ________ (polar, nonpolar) alkyl chain. In the case of “normal” soap, the head- group is a ___CARBOXYLATE__ anion. Indicate where we would find the associated cations (i.e., sodium cations; draw a few in the scheme). Water is on the __________ (inside, outside) of this micelle and fats will accumulate on the _________ (inside, outside) of this micelle. -

Stereochemistry of Bruice's Organic Chemistry Before Embarking on Comprehending This Summary
Enantioselective synthesis It helps if you review the concepts in Chapter 5: Stereochemistry of Bruice's Organic Chemistry before embarking on comprehending this summary Most of the molecules on earth are chiral, such as amino acids and carbohydrates. Enantioselective synthesis is an important means by which enantiopure chiral molecules may be obtained for study and medicine. The study of enantioselective synthesis has evolved from issues of diastereoselectivity, through auxiliary-based methods for the synthesis of enantiomerically pure compounds (diastereoselectivity followed by separation and auxiliary cleavage), to asymmetric catalysis. In the latter technology, enantiomers are the products, and highly selective reactions allow preparation - in a single step - of chiral substances in high enantiomeric excess (ee) for many reaction types. Enantioselective synthesis, also known as asymmetric synthesis, is organic synthesis that introduces one or more new and desired elements of chirality. This methodology is important in the field of pharmaceuticals because the different enantiomers or diastereomers of a molecule often have different biological activity. See examples from Chapter 5 of Bruice's Organic Chemistry. Methodologies of Asymmetric Synthesis Chiral pool synthesis Chiral pool synthesis is the easiest approach: A chiral starting material is manipulated through successive reactions using achiral reagents that retain its chirality to obtain the desired target molecule. This is especially attractive for target molecules having the similar chirality to a relatively inexpensive naturally occurring building-block such as a sugar or amino acid. However, the number of possible reactions the molecule can undergo is restricted, and lengthy synthetic routes may be required. Also, this approach requires a stoichiometric amount of the enantiopure starting material, which may be rather expensive if not occurring in nature, whereas chiral catalysis requires only a catalytic amount of chiral material. -

NMR and Stereochemistry Chem 4010/5326: Organic Spectroscopic Analysis
NMR and Stereochemistry Chem 4010/5326: Organic Spectroscopic Analysis © 2015 Andrew Harned General flow for solving structures C10H20O Molecular weight/formula (MS) Exact Mass: 156.1514 Molecular Weight: 156.2652 Functional groups (IR, NMR) Carbon connectivities (substructures) (NMR) Positions of functional groups within framework (gross structure) (2D NMR, coupling constants) How can this Stereochemical issues be solved??? Relative Stereochemistry (Diastereomers) Can be determined with many of the tools we have already discussed, along with some new ones Bifulco, G.; Dambruoso, P.; Gomex-Paloma, L.; Riccio, R. Chem. Rev. 2007, 107, 3744. NMR Spectroscopy Strategies for determining relative stereochemistry Chemical Shifts – Diastereotopic protons will have different chemical shifts, this will only tell you that diastereomers are present, cannot necessarily tell which is which by inspection only by comparison to known structures – Spatial orientation may place certain protons in shielding/deshielding portions of functional groups Coupling Constants – In acyclic systems, usually cannot tell which is which by inspection – Often must convert to rigid/cyclic structure Both require some knowledge of 3D structure –> Make model(s) NMR Spectroscopy Proximity of Protons Nuclear Overhauser Effect (nOe) – Through space interactions between nuclei, whether or not they are directly coupled – Magnitude decreases as inverse of sixth power of distance – Strongly irradiate one, get larger # in excited state – The others then shift to lower state to compensate -
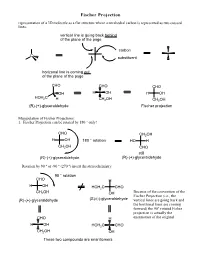
Fischer Projection Representation of a 3D Molecule As a Flat Structure Where a Tetrahedral Carbon Is Represented As Two Crossed Lines
Fischer Projection representation of a 3D molecule as a flat structure where a tetrahedral carbon is represented as two crossed lines. vertical line is going back behind of the plane of the page carbon C substituent horizonal line is coming out of the plane of the page CHO CHO CHO OH H OH H OH HOH2C H CH2OH CH2OH (R)-(+)-glyceraldehyde Fischer projection Manipulation of Fischer Projections: 1. Fischer Projection can be rotated by 180 ° only! CHO CH2OH H OH 180 ° rotation HO H CH2OH CHO still (R)-(+)-glyceraldehyde (R)-(+)-glyceraldehyde Rotation by 90 ° or -90 ° (270 °) invert the stereochemistry 90 ° rotation CHO H H OH HOH2C CHO CH2OH OH Because of the convention of the Fischer Projection (i.e., the (R)-(+)-glyceraldehyde (S)-(-)-glyceraldehyde vertical lines are going back and the horizonal lines are coming forward) the 90° rotated Fisher projection is actually the CHO H enantiomer of the original H OH HOH2C CHO CH2OH OH These two compounds are enantiomers 2. If one group of the Fischer projection is held steady, the other groups can be rotated either clockwise or counter clockwise. hold this group steady CHO CHO H OH HOH2C H CH2OH OH still (R)-(+)-glyceraldehyde (R)-(+)-glyceraldehyde Assigning R and S-configurations to Fischer projections 1. Assign priorities to the four substituents according to the Cahn-Ingold-Prelog convention. 2. Perform the two allowed manipulations of the Fischer projection to place the lowest priority group on one of the vertical positions (either top or bottom) 3. If the priorities of the other three groups (1-2-3) proceed clockwise, the stereogenic center is assigned as R. -

Asymmetric Synthesis
Chapter 45 — Asymmetric synthesis - Pure enantiomers from Nature: the chiral pool and chiral induction - Asymmetric synthesis: chiral auxiliaries Enolate alkylation Aldol reaction - Enantiomeric excess (ee) - Asymmetric synthesis: chiral reagents and catalysts CBS reagent for chiral reductions Sharpless asymmetric epoxidation Synthesizing pure enantiomers starting from Nature’s chiral pool AcO OH HO B O O HO – O O HO + O N OH H3N OH HO OH O H … AcO HO OH O OH Sulcatol - insect pheremone HO Synthesis from the chiral pool — chiral induction turns one stereocenter into many 40 steps Me HO H Me H O O OH O O OBn O * * Only one H O HO chiral reagent H H Me OBn Me Deoxyribose Fragment of Brevetoxin B Asymmetric synthesis 1. Producing a new stereogenic centre on an achiral molecule makes two enantiomeric transition states of equal energy… and therefore two enantiomeric products in equal amounts. O O 1 1 Nu R R2 R R2 Nu E OH OH 1 O 1 Nu R R Nu R2 R2 1 R R2 O Ph OH HO Ph PhLi + N N N Asymmetric synthesis 2. O 1 R R* Nu When there is an existing O chiral centre, the two possible TS’s are diastereomeric and E 1 Nu R R* can be of different energy. Thus one isomer of the new stereogenic centre can be OH O OH produced in a larger amount. 1 1 R Nu Nu R *R 1 R* R R* HO Ph O O O Ph OH PhLi PhLi Ph Ph N N N N N Ph N N N N N OH Ph O O O Ph OH Ph A removable chiral centre… synthesis with chiral auxiliaries O ??? O R R 1) Add a chiral 3) Remove the auxiliary chiral auxiliary O O R* R* 2) Add the new stereocentre via chiral induction Enantiopure oxazolidinones as chiral auxiliaries 1.