Traumatic Hemothorax Caused by Thoracic Wall and Intrathoracic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

081999 Disseminated Intravascular Coagulation
The New England Journal of Medicine Current Concepts Systemic activation+ of coagulation DISSEMINATED INTRAVASCULAR COAGULATION Intravascular+ Depletion of platelets+ deposition of fibrin and coagulation factors MARCEL LEVI, M.D., AND HUGO TEN CATE, M.D. Thrombosis of small+ Bleeding and midsize vessels+ ISSEMINATED intravascular coagulation is and organ failure characterized by the widespread activation Dof coagulation, which results in the intravas- Figure 1. The Mechanism of Disseminated Intravascular Coag- cular formation of fibrin and ultimately thrombotic ulation. occlusion of small and midsize vessels.1-3 Intravascu- Systemic activation of coagulation leads to widespread intra- lar coagulation can also compromise the blood sup- vascular deposition of fibrin and depletion of platelets and co- agulation factors. As a result, thrombosis of small and midsize ply to organs and, in conjunction with hemodynam- vessels may occur, contributing to organ failure, and there may ic and metabolic derangements, may contribute to be severe bleeding. the failure of multiple organs. At the same time, the use and subsequent depletion of platelets and coag- ulation proteins resulting from the ongoing coagu- lation may induce severe bleeding (Fig. 1). Bleeding may be the presenting symptom in a patient with disseminated intravascular coagulation, a factor that can complicate decisions about treatment. TABLE 1. COMMON CLINICAL CONDITIONS ASSOCIATED WITH DISSEMINATED ASSOCIATED CLINICAL CONDITIONS INTRAVASCULAR COAGULATION. AND INCIDENCE Sepsis Infectious Disease Trauma Serious tissue injury Disseminated intravascular coagulation is an ac- Head injury Fat embolism quired disorder that occurs in a wide variety of clin- Cancer ical conditions, the most important of which are listed Myeloproliferative diseases in Table 1. -

What Everyone Should Know to Stop Bleeding After an Injury
What Everyone Should Know to Stop Bleeding After an Injury THE HARTFORD CONSENSUS The Joint Committee to Increase Survival from Active Shooter and Intentional Mass Casualty Events was convened by the American College of Surgeons in response to the growing number and severity of these events. The committee met in Hartford Connecticut and has produced a number of documents with rec- ommendations. The documents represent the consensus opinion of a multi-dis- ciplinary committee involving medical groups, the military, the National Security Council, Homeland Security, the FBI, law enforcement, fire rescue, and EMS. These recommendations have become known as the Hartford Consensus. The overarching principle of the Hartford Consensus is that no one should die from uncontrolled bleeding. The Hartford Consensus recommends that all citizens learn to stop bleeding. Further information about the Hartford Consensus and bleeding control can be found on the website: Bleedingcontrol.org 2 SAVE A LIFE: What Everyone Should Know to Stop Bleeding After an Injury Authors: Peter T. Pons, MD, FACEP Lenworth Jacobs, MD, MPH, FACS Acknowledgements: The authors acknowledge the contributions of Michael Cohen and James “Brooks” Hart, CMI to the design of this manual. Some images adapted from Adam Wehrle, EMT-P and NAEMT. © 2017 American College of Surgeons CONTENTS SECTION 1 3 ■ Introduction ■ Primary Principles of Trauma Care Response ■ The ABCs of Bleeding SECTION 2 5 ■ Ensure Your Own Safety SECTION 3 6 ■ A – Alert – call 9-1-1 SECTION 4 7 ■ B – Bleeding – find the bleeding injury SECTION 5 9 ■ C – Compress – apply pressure to stop the bleeding by: ■ Covering the wound with a clean cloth and applying pressure by pushing directly on it with both hands, OR ■Using a tourniquet, OR ■ Packing (stuff) the wound with gauze or a clean cloth and then applying pressure with both hands SECTION 6 13 ■ Summary 2 SECTION 1: INTRODUCTION Welcome to the Stop the Bleed: Bleeding Control for the Injured information booklet. -

Neurologic Deterioration Secondary to Unrecognized Spinal Instability Following Trauma–A Multicenter Study
SPINE Volume 31, Number 4, pp 451–458 ©2006, Lippincott Williams & Wilkins, Inc. Neurologic Deterioration Secondary to Unrecognized Spinal Instability Following Trauma–A Multicenter Study Allan D. Levi, MD, PhD,* R. John Hurlbert, MD, PhD,† Paul Anderson, MD,‡ Michael Fehlings, MD, PhD,§ Raj Rampersaud, MD,§ Eric M. Massicotte, MD,§ John C. France, MD, Jean Charles Le Huec, MD, PhD,¶ Rune Hedlund, MD,** and Paul Arnold, MD†† Study Design. A retrospective study was undertaken their neurologic injury. The most common reason for the that evaluated the medical records and imaging studies of missed injury was insufficient imaging studies (58.3%), a subset of patients with spinal injury from large level I while only 33.3% were a result of misread radiographs or trauma centers. 8.3% poor quality radiographs. The incidence of missed Objective. To characterize patients with spinal injuries injuries resulting in neurologic injury in patients with who had neurologic deterioration due to unrecognized spine fractures or strains was 0.21%, and the incidence as instability. a percentage of all trauma patients evaluated was 0.025%. Summary of Background Data. Controversy exists re- Conclusions. This multicenter study establishes that garding the most appropriate imaging studies required to missed spinal injuries resulting in a neurologic deficit “clear” the spine in patients suspected of having a spinal continue to occur in major trauma centers despite the column injury. Although most bony and/or ligamentous presence of experienced personnel and sophisticated im- spine injuries are detected early, an occasional patient aging techniques. Older age, high impact accidents, and has an occult injury, which is not detected, and a poten- patients with insufficient imaging are at highest risk. -

Spontaneous Hemothorax Caused by a Ruptured Intercostal Artery Aneurysm in Von Recklinghausen’S Neurofibromatosis
W.C. Chang, H.H. Hsu, H. Chang, et al BRIEF COMMUNICATION SPONTANEOUS HEMOTHORAX CAUSED BY A RUPTURED INTERCOSTAL ARTERY ANEURYSM IN VON RECKLINGHAUSEN’S NEUROFIBROMATOSIS Wei-Chou Chang,1 Hsian-He Hsu,1 Hung Chang,2 and Cheng-Yu Chen1 Abstract: Aneurysms arising from an intercostal artery are very rare vascular malformations in von Recklinghausen’s neurofibromatosis, which often have a silent clinical presentation and are difficult to diagnose before rupture. We report a case of von Recklinghausen’s neurofibromatosis with massive hemothroax caused by spontaneous rupture of an intercostal artery aneurysm in a 29-year-old man. The diagnosis was eventually confirmed by percutaneous angiography and treated with endovascular embolization. During a 10-month follow-up period, the patient had a satisfactory recovery. This case illustrates that angiography and possible endovascular embolization should be the first strategy in managing hemothorax in patients with von Recklinghausen’s disease. Key words: Aneurysm, ruptured; Embolization, therapeutic; Hemothorax; Neurofibromatosis 1; Thoracic arteries J Formos Med Assoc 2005;104:286-9 Von Recklinghausen’s neurofibromatosis, also named neurofibromatosis type 1 (NF-1), is a well-recognized Case Report entity that is characterized by numerous neuro- fibromas, spots of abnormal cutaneous pigmentation, A 29-year-old man presented at the emergency room and a variety of other dysplastic abnormalities of with sudden onset shortness of breath and severe the skin, nervous system, bones, endocrine organs and retrosternal pain radiating to his back. One week prior blood vessels.1,2 Vascular abnormalities in patients to admission, dull discomfort upon breathing was with NF-1 have been described for large, medium- noted, which was not affected by position or physical sized and small muscular arteries in the intracranial, activity. -
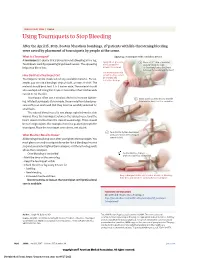
Using Tourniquets to Stop Bleeding
JAMA PATIENT PAGE | Trauma Using Tourniquets to Stop Bleeding After the April 15, 2013, Boston Marathon bombings, 27 patients with life-threatening bleeding were saved by placement of tourniquets by people at the scene. What Is a Tourniquet? Applying a tourniquet with a windlass device A tourniquet is a device that is placed around a bleeding arm or leg. Apply direct pressure 1 Place a 2-3” strip of material Tourniquets work by squeezing large blood vessels. The squeezing to the wound for about 2” from the edge helps stop blood loss. at least 15 minutes. of the wound over a long bone between the wound and the heart. Use a tourniquet only How Do I Put a Tourniquet On? when bleeding cannot be stopped and Tourniquets can be made out of any available material. For ex- is life threatening. ample, you can use a bandage, strip of cloth, or even a t-shirt. The material should be at least 2 to 3 inches wide. The material should also overlap itself. Using thin straps or material less than 2 inches wide can rip or cut the skin. Tourniquets often use a windlass device to increase tighten- 2 Insert a stick or other strong, straight ing. Inflated tourniquets (for example, those made from blood pres- item into the knot to act as a windlass. sure cuffs) can work well. But they must be carefully watched for small leaks. The injured blood vessel is not always right below the skin wound. Place the tourniquet between the injured vessel and the heart, about 2 inches from the closest wound edge. -

Fat Embolism Syndrome – a Qualitative Review of Its Incidence, Presentation, Pathogenesis and Management
2-RA_OA1 3/24/21 6:00 PM Page 1 Malaysian Orthopaedic Journal 2021 Vol 15 No 1 Timon C, et al doi: https://doi.org/10.5704/MOJ.2103.001 REVIEW ARTICLE Fat Embolism Syndrome – A Qualitative Review of its Incidence, Presentation, Pathogenesis and Management Timon C, MCh, Keady C, MSc, Murphy CG, FRCS Department of Trauma and Orthopaedics, Galway University Hospitals, Galway, Ireland This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited Date of submission: 12th November 2020 Date of acceptance: 05th March 2021 ABSTRACT DEFINITION AND INTRODUCTION Fat Embolism Syndrome (FES) is a poorly defined clinical Fat embolism 1 occurs when fat enters the circulation, this fat phenomenon which has been attributed to fat emboli entering can embolise and may or may not produce clinical the circulation. It is common, and its clinical presentation manifestations. may be either subtle or dramatic and life threatening. This is a review of the history, causes, pathophysiology, FES is a poorly defined clinical phenomenon which has been presentation, diagnosis and management of FES. FES mostly attributed to fat emboli entering the circulation. It classically occurs secondary to orthopaedic trauma; it is less frequently presents with respiratory, neurological and dermatological associated with other traumatic and atraumatic conditions. features. It typically occurs after long-bone fractures and There is no single test for diagnosing FES. Diagnosis of FES total hip arthroplasty, less frequently it is caused by burns is often missed due to its subclinical presentation and/or and soft tissue injuries 2. -

ER Guide to Bleeding Disorders
Bleeding disorders ER guide to bleeding disorders 1 Table of contents 4 General Guidelines 4–5 national Hemophilia Foundation guidelines 5–10 Treatment options 10 HemopHilia a Name:__________________________________________________________________________________________________ 10–11 national Hemophilia Foundation guidelines Address:________________________________________________________________________________________________ 12 dosage chart Phone:__________________________________________________________________________________________________ 14–15 Treatment products 16 HemopHilia B In case of emergency, contact: ______________________________________________________________________________ 16 national Hemophilia Foundation guidelines Relation to patient:________________________________________________________________________________________ 17 dosage chart 18 Treatment products 19 HemopHilia a or B with inHiBiTors Diagnosis: Hemophilia A: Mild Moderate Severe 20 national Hemophilia Foundation guidelines Inhibitors Inhibitors Bethesda units (if known) ____________________________________ 21 Treatment products Hemophilia B: Mild Moderate Severe 22–23 Von willeBrand disease Inhibitors Inhibitors Bethesda units (if known) ____________________________________ 23–24 national Hemophilia Foundation guidelines von Willebrand disease: Type 1 Type 2 Type 3 Platelet type 25 Treatment products 27 Bibliography Preferred product:_________________________________________________________________________________________ Dose for life-threatening -

UHS Adult Major Trauma Guidelines 2014
Adult Major Trauma Guidelines University Hospital Southampton NHS Foundation Trust Version 1.1 Dr Andy Eynon Director of Major Trauma, Consultant in Neurosciences Intensive Care Dr Simon Hughes Deputy Director of Major Trauma, Consultant Anaesthetist Dr Elizabeth Shewry Locum Consultant Anaesthetist in Major Trauma Version 1 Dr Andy Eynon Dr Simon Hughes Dr Elizabeth ShewryVersion 1 1 UHS Adult Major Trauma Guidelines 2014 NOTE: These guidelines are regularly updated. Check the intranet for the latest version. DO NOT PRINT HARD COPIES Please note these Major Trauma Guidelines are for UHS Adult Major Trauma Patients. The Wessex Children’s Major Trauma Guidelines may be found at http://staffnet/TrustDocsMedia/DocsForAllStaff/Clinical/Childr ensMajorTraumaGuideline/Wessexchildrensmajortraumaguid eline.doc NOTE: If you are concerned about a patient under the age of 16 please contact SORT (02380 775502) who will give valuable clinical advice and assistance by phone to the Trauma Unit and coordinate any transfer required. http://www.sort.nhs.uk/home.aspx Please note current versions of individual University Hospital South- ampton Major Trauma guidelines can be found by following the link below. http://staffnet/TrustDocuments/Departmentanddivision- specificdocuments/Major-trauma-centre/Major-trauma-centre.aspx Version 1 Dr Andy Eynon Dr Simon Hughes Dr Elizabeth Shewry 2 UHS Adult Major Trauma Guidelines 2014 Contents Please ‘control + click’ on each ‘Section’ below to link to individual sections. Section_1: Preparation for Major Trauma Admissions -

Thrombocytopenia.Pdf
THROMBOCYTOPENIA DIFFERENTIAL DIAGNOSIS FALSELY LOW PLATELET COUNT In vitro platelet clumping caused by EDTA-dependent agglutinins Giant platelets COMMON CAUSES OF THROMBOCYTOPENIA Pregnancy (gestational thrombocytopenia, preeclampsia) Drug-induced thrombocytopenia (i.e., heparin, quinidine, quinine, and sulfonamides) Viral infections (ie. HIV, rubella, infectious mononucleosis) Hypersplenism due to chronic liver disease Dilutional (massive transfusion) OTHER CAUSES OF THROMBOCYTOPENIA Myelodysplasia Congenital thrombocytopenia Thrombotic thrombocytopenic purpura (TTP) -hemolytic-uremic syndrome (HUS) Chronic disseminated intravascular coagulation (DIC) Autoimmune diseases, such as systemic lupus erythematosus-associated lymphoproliferative disorders (CLL and NHL) Sepsis Idiopathic thrombocytopenic purpura (ITP)* DIFFERENTIAL FOR THROMBOCYTOPENIA BASED ON CLINICAL SETTING CLINICAL SETTING DIFFERENTIAL DIAGNOSES Cardiac surgery Cardiopulmonary bypass, HIT, dilutional thrombocytopenia, PTP Interventional cardiac Abciximab or other IIb/IIIa blockers, HIT procedure Sepsis syndrome DIC, ehrlichiosis, sepsis, hemophagocytosis syndrome, drug-induced, misdiagnosed TTP, mechanical ventilation, pulmonary artery catheters Pulmonary failure DIC, hantavirus pulmonary syndrome, mechanical ventilation, pulmonary artery catheters Mental status TTP, ehrlichiosis changes/seizures Renal failure TTP, Dengue, HIT, DIC, HUS Continuous hemofiltration HIT, consumption by filter and tubing Cardiac failure HIT, drug-induced, pulmonary artery catheter Post-surgery -
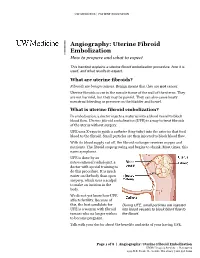
Uterine Fibroid Embolization Procedure, How It Is Used, and What Results to Expect
UW MEDICINE | PATIENT EDUCATION | | Angiography: Uterine Fibroid | Embolization | How to prepare and what to expect This handout explains a uterine fibroid embolization procedure, how it is used, and what results to expect. What are uterine fibroids? Fibroids are benign tumors. Benign means that they are not cancer. Uterine fibroids occur in the muscle tissue of the wall of the uterus. They are not harmful, but they may be painful. They can also cause heavy menstrual bleeding or pressure on the bladder and bowel. What is uterine fibroid embolization? In embolization, a doctor injects a material into a blood vessel to block blood flow. Uterine fibroid embolization (UFE) is a way to treat fibroids DRAFTof the uterus without surgery. UFE uses X-rays to guide a catheter (tiny tube) into the arteries that feed blood to the fibroid. Small particles are then injected to block blood flow. With its blood supply cut off, the fibroid no longer receives oxygen and nutrients. The fibroid stops growing and begins to shrink. Most times, this eases symptoms. UFE is done by an interventional radiologist, a doctor with special training to do this procedure. It is much easier on the body than open surgery, which uses a scalpel to make an incision in the body. We do not yet know how UFE affects fertility. Because of this, the best candidate for During UFE, small particles are injected UFE is a woman with fibroid into blood vessels to block blood flow to tumors who no longer wishes the fibroid. to become pregnant. Talk with your doctor about the benefits and risks of your having UFE. -

Ten Patient Stories Illustrating the Extraordinarily Diverse Clinical Features of Patients with Thrombotic Thrombocytopenic Purpura and Severe ADAMTS13 Deficiency
Journal of Clinical Apheresis 27:302–311 (2012) Ten Patient Stories Illustrating the Extraordinarily Diverse Clinical Features of Patients With Thrombotic Thrombocytopenic Purpura and Severe ADAMTS13 Deficiency James N. George,* Qiaofang Chen, Cassie C. Deford, and Zayd Al-Nouri Department of Biostatistics and Epidemiology, College of Public Health, Department of Medicine, College of Medicine, The University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma Patients with thrombotic thrombocytopenic purpura (TTP) and severe ADAMTS13 deficiency are often consid- ered to have typical clinical features. However, our experience is that there is extraordinary diversity of the pre- senting features and the clinical courses of these patients. This diversity is illustrated by descriptions of 10 patients. The patients illustrate that ADAMTS13 activity may be normal initially but severely deficient in subse- quent episodes. Patients with established diagnoses of systemic infection as the cause of their clinical features may have undetectable ADAMTS13 activity. Patients may have a prolonged prodrome of mild symptoms with only microangiopathic hemolytic anemia and thrombocytopenia or they may have the sudden onset of critical ill- ness with multiple organ involvement. Patients may die rapidly or recover rapidly; they may require minimal treatment or extensive and prolonged treatment. Patients may have acute and severe neurologic abnormalities before microangiopathic hemolytic anemia and thrombocytopenia occur. Patients may have concurrent TTP and systemic lupus erythematosus. Patients may have hereditary ADAMTS13 deficiency as the etiology of their TTP rather than acquired autoimmune ADAMTS13 deficiency. These patients’ stories illustrate the clinical spectrum of TTP with ADAMTS13 deficiency and emphasize the difficulties of clinical diagnosis. J. Clin. -

Guidelines for the Management of Severe Traumatic Brain Injury 4Th Edition
Guidelines for the Management of Severe Traumatic Brain Injury 4th Edition Nancy Carney, PhD Oregon Health & Science University, Portland, OR Annette M. Totten, PhD Oregon Health & Science University, Portland, OR Cindy O'Reilly, BS Oregon Health & Science University, Portland, OR Jamie S. Ullman, MD Hofstra North Shore-LIJ School of Medicine, Hempstead, NY Gregory W. J. Hawryluk, MD, PhD University of Utah, Salt Lake City, UT Michael J. Bell, MD University of Pittsburgh, Pittsburgh, PA Susan L. Bratton, MD University of Utah, Salt Lake City, UT Randall Chesnut, MD University of Washington, Seattle, WA Odette A. Harris, MD, MPH Stanford University, Stanford, CA Niranjan Kissoon, MD University of British Columbia, Vancouver, BC Andres M. Rubiano, MD El Bosque University, Bogota, Colombia; MEDITECH Foundation, Neiva, Colombia Lori Shutter, MD University of Pittsburgh, Pittsburgh, PA Robert C. Tasker, MBBS, MD Harvard Medical School & Boston Children’s Hospital, Boston, MA Monica S. Vavilala, MD University of Washington, Seattle, WA Jack Wilberger, MD Drexel University, Pittsburgh, PA David W. Wright, MD Emory University, Atlanta, GA Jamshid Ghajar, MD, PhD Stanford University, Stanford, CA Reviewed for evidence-based integrity and endorsed by the American Association of Neurological Surgeons and the Congress of Neurological Surgeons. September 2016 TABLE OF CONTENTS PREFACE ...................................................................................................................................... 5 ACKNOWLEDGEMENTS .............................................................................................................................................