Familial Long QT Syndrome and Late Development of Dilated Cardiomyopathy in a Child with a KCNQ1 Mutation: a Case Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Non Commercial Use Only
Cardiogenetics 2017; volume 7:6304 Sudden death in a young patient with atrial fibrillation Case Report Correspondence: María Angeles Espinosa Castro, Inherited Cardiovascular Disease A 22-year-old man suffered a sudden Program, Cardiology Department, Gregorio María Tamargo, cardiac arrest without previous symptoms Marañón Hospital, Dr. Esquerdo, 46, 28007, María Ángeles Espinosa, while he was at rest, waiting for a subway Madrid, Spain. Víctor Gómez-Carrillo, Miriam Juárez, train. Cardiopulmonary resuscitation was Tel.: +34.91.586.82.90. immediately started using an Automated E-mail: [email protected] Francisco Fernández-Avilés, External Defibrillation that identified the Raquel Yotti Key words: KCNQ1; mutation; channelopa- presence of ventricular fibrillation and thy; sudden cardiac death; atrial fibrillation. Inherited Cardiovascular Disease delivered a shock. Return of spontaneous Program, Cardiology Department, circulation was achieved after three Contributions: MT, acquisition and interpreta- Gregorio Marañón Hospital, Madrid, attempts, being atrial fibrillation (AF) the tion of data for the work, ensuring that ques- Spain patient’s rhythm at this point (Figure 1). tions related to the accuracy or integrity of any He was admitted to our Cardiovascular part of the work is appropriately investigated Intensive Care Unit and therapeutic and resolved; MAE, conception of the work, hypothermia was performed over a period critical revision of the intellectual content, final approval of the version to be published, Abstract of 24 h. After completing hypothermia, ensuring that questions related to the accuracy rewarming, and another 24 h of controlled of any part of the work is appropriately inves- Sudden cardiac death (SCD) in young normothermia the patient awakened with no tigated and resolved; VG-C, acquisition and patients without structural heart disease is residual neurologic damage. -

Myocarditis and Cardiomyopathy
CE: Tripti; HCO/330310; Total nos of Pages: 6; HCO 330310 REVIEW CURRENT OPINION Myocarditis and cardiomyopathy Jonathan Buggey and Chantal A. ElAmm Purpose of review The aim of this study is to summarize the literature describing the pathogenesis, diagnosis and management of cardiomyopathy related to myocarditis. Recent findings Myocarditis has a variety of causes and a heterogeneous clinical presentation with potentially life- threatening complications. About one-third of patients will develop a dilated cardiomyopathy and the pathogenesis is a multiphase, mutlicompartment process that involves immune activation, including innate immune system triggered proinflammatory cytokines and autoantibodies. In recent years, diagnosis has been aided by advancements in cardiac MRI, and in particular T1 and T2 mapping sequences. In certain clinical situations, endomyocardial biopsy (EMB) should be performed, with consideration of left ventricular sampling, for an accurate diagnosis that may aid treatment and prognostication. Summary Although overall myocarditis accounts for a minority of cardiomyopathy and heart failure presentations, the clinical presentation is variable and the pathophysiology of myocardial damage is unique. Cardiac MRI has significantly improved diagnostic abilities, but endomyocardial biopsy remains the gold standard. However, current treatment strategies are still focused on routine heart failure pharmacotherapies and supportive care or cardiac transplantation/mechanical support for those with end-stage heart failure. Keywords cardiac MRI, cardiomyopathy, endomyocardial biopsy, myocarditis INTRODUCTION prevalence seen in children and young adults aged Myocarditis refers to inflammation of the myocar- 20–30 years [1]. dium and may be caused by infectious agents, systemic diseases, drugs and toxins, with viral infec- CAUSE tions remaining the most common cause in the developed countries [1]. -

ICD-10-CM Documentation and Coding Best Practices
ICD-10-CM Documentation and Coding Best Practices Cardiomyopathy Cardiomyopathy refers to diseases of the heart muscle, which can become enlarged, thick or rigid. In rare cases, cardiac muscle tissue can be replaced with scar tissue. As the condition worsens, the heart becomes weaker and less able to pump blood through the body or to maintain a normal electrical rhythm. Causes Risk factors that can increase the possibility of developing cardiomyopathy include: coronary artery disease, a history of heart attack (s), viral infections that cause heart inflammation, long-term hypertension or alcoholism, obesity, and diabetes to name a few. However, in most cases the exact cause is usually unknown (called primary or idiopathic cardiomyopathy). Symptoms Some patients will never have symptoms; others will not develop them until later in the disease. Symptoms can include: • Fatigue • Shortness of b reath or trouble breathing (dyspnea) • Dizziness, lightheadedness or fainting • Swelling in the ankles, feet, legs, abdomen and neck veins Treatment Treatment depends on the type of cardiomyopathy, the severity of symptoms, and the patient’s age and overall health. • Lifestyle changes can help manage condition(s) that may be causing cardiomyopathy. Recommendations include: o Consuming a heart healthy diet, engaging in physical activity, losing excess weight, giving up smoking, avoiding alcohol and illegal drugs, getting enough sleep, and reducing stress • Medicines may be p rescribed to: o Lower blood pressure (ACE inhibitors, angiotensin II receptor blo ckers, beta blockers, calcium channel blockers ) o Slow the heart rate (beta blockers, calcium channel blockers, digoxin) o Prevent arrhythmias (antiarrhythmics) o Remove excess fluid and sodium (diuretics) o Prevent blood clots (anticoagulants) • Alcohol septal ablation • Surgery o Septal myectomy – option for severe cases of obstructive hypertrophic cardiomyopathy Surgically implanted devices o . -
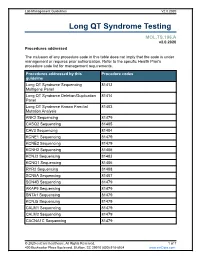
Long QT Syndrome Testing
Lab Management Guidelines V2.0.2020 Long QT Syndrome Testing MOL.TS.196.A v2.0.2020 Procedures addressed The inclusion of any procedure code in this table does not imply that the code is under management or requires prior authorization. Refer to the specific Health Plan's procedure code list for management requirements. Procedures addressed by this Procedure codes guideline Long QT Syndrome Sequencing 81413 Multigene Panel Long QT Syndrome Deletion/Duplication 81414 Panel Long QT Syndrome Known Familial 81403 Mutation Analysis ANK2 Sequencing 81479 CASQ2 Sequencing 81405 CAV3 Sequencing 81404 KCNE1 Sequencing 81479 KCNE2 Sequencing 81479 KCNH2 Sequencing 81406 KCNJ2 Sequencing 81403 KCNQ1 Sequencing 81406 RYR2 Sequencing 81408 SCN5A Sequencing 81407 SCN4B Sequencing 81479 AKAP9 Sequencing 81479 SNTA1 Sequencing 81479 KCNJ5 Sequencing 81479 CALM1 Sequencing 81479 CALM2 Sequencing 81479 CACNA1C Sequencing 81479 © 2020 eviCore healthcare. All Rights Reserved. 1 of 7 400 Buckwalter Place Boulevard, Bluffton, SC 29910 (800) 918-8924 www.eviCore.com Lab Management Guidelines V2.0.2020 What is Long QT syndrome Definition Long QT Syndrome (LQTS) is caused by mutations in a number of genes, most of which are related to the functioning of sodium or potassium ion channels in the heart.1 Testing may offer prognostic information in some cases, as specific genes and even specific mutations within those genes may have some correlation to risk for sudden death, effectiveness of beta-blocker therapy, and preventive strategies.1-4 Signs and symptoms of long QT syndrome (LQTS) are variable, but may include a prolonged QT interval on an electrocardiogram, torsades de pointes, syncope, seizures, cardiac arrest, and sudden cardiac death.1,2 Many patients with LQTS can be largely asymptomatic, with cardiac arrest or sudden cardiac death as the first and only symptom. -

Drugs and Life-Threatening Ventricular Arrhythmia Risk: Results from the DARE Study Cohort
Open Access Research BMJ Open: first published as 10.1136/bmjopen-2017-016627 on 16 October 2017. Downloaded from Drugs and life-threatening ventricular arrhythmia risk: results from the DARE study cohort Abigail L Coughtrie,1,2 Elijah R Behr,3,4 Deborah Layton,1,2 Vanessa Marshall,1 A John Camm,3,4,5 Saad A W Shakir1,2 To cite: Coughtrie AL, Behr ER, ABSTRACT Strengths and limitations of this study Layton D, et al. Drugs and Objectives To establish a unique sample of proarrhythmia life-threatening ventricular cases, determine the characteristics of cases and estimate ► The Drug-induced Arrhythmia Risk Evaluation study arrhythmia risk: results from the the contribution of individual drugs to the incidence of DARE study cohort. BMJ Open has allowed the development of a cohort of cases of proarrhythmia within these cases. 2017;7:e016627. doi:10.1136/ proarrhythmia. Setting Suspected proarrhythmia cases were referred bmjopen-2017-016627 ► These cases have provided crucial safety by cardiologists across England between 2003 and 2011. information, as well as underlying clinical and ► Prepublication history for Information on demography, symptoms, prior medical and genetic data. this paper is available online. drug histories and data from hospital notes were collected. ► Only patients who did not die as a result of the To view these files please visit Participants Two expert cardiologists reviewed data the journal online (http:// dx. doi. proarrhythmia could be included. for 293 referred cases: 130 were included. Inclusion org/ 10. 1136/ bmjopen- 2017- ► Referral of cases by cardiologists alone may have criteria were new onset or exacerbation of pre-existing 016627). -

Atrial Fibrillation (ATRIA) Study
European Journal of Human Genetics (2014) 22, 297–306 & 2014 Macmillan Publishers Limited All rights reserved 1018-4813/14 www.nature.com/ejhg REVIEW Atrial fibrillation: the role of common and rare genetic variants Morten S Olesen*,1,2,4, Morten W Nielsen1,2,4, Stig Haunsø1,2,3 and Jesper H Svendsen1,2,3 Atrial fibrillation (AF) is the most common cardiac arrhythmia affecting 1–2% of the general population. A number of studies have demonstrated that AF, and in particular lone AF, has a substantial genetic component. Monogenic mutations in lone and familial AF, although rare, have been recognized for many years. Presently, mutations in 25 genes have been associated with AF. However, the complexity of monogenic AF is illustrated by the recent finding that both gain- and loss-of-function mutations in the same gene can cause AF. Genome-wide association studies (GWAS) have indicated that common single-nucleotide polymorphisms (SNPs) have a role in the development of AF. Following the first GWAS discovering the association between PITX2 and AF, several new GWAS reports have identified SNPs associated with susceptibility of AF. To date, nine SNPs have been associated with AF. The exact biological pathways involving these SNPs and the development of AF are now starting to be elucidated. Since the first GWAS, the number of papers concerning the genetic basis of AF has increased drastically and the majority of these papers are for the first time included in a review. In this review, we discuss the genetic basis of AF and the role of both common and rare genetic variants in the susceptibility of developing AF. -

An Uncommon Clinical Presentation of Relapsing Dilated Cardiomyopathy with Identification of Sequence Variations in MYNPC3, KCNH2 and Mitochondrial Trna Cysteine M
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by George Washington University: Health Sciences Research Commons (HSRC) Himmelfarb Health Sciences Library, The George Washington University Health Sciences Research Commons Pediatrics Faculty Publications Pediatrics 6-2015 An uncommon clinical presentation of relapsing dilated cardiomyopathy with identification of sequence variations in MYNPC3, KCNH2 and mitochondrial tRNA cysteine M. J. Guillen Sacoto Kimberly A. Chapman George Washington University D. Heath M. B. Seprish Dina Zand George Washington University Follow this and additional works at: http://hsrc.himmelfarb.gwu.edu/smhs_peds_facpubs Part of the Pediatrics Commons Recommended Citation Guillen Sacoto, M.J., Chapman, K.A., Heath, D., Seprish, M.B., Zand, D.J. (2015). An uncommon clinical presentation of relapsing dilated cardiomyopathy with identification of sequence variations in MYNPC3, KCNH2 and mitochondrial tRNA cysteine. Molecular Genetics and Metabolism Reports, 3, 47-54. doi:10.1016/j.ymgmr.2015.03.007 This Journal Article is brought to you for free and open access by the Pediatrics at Health Sciences Research Commons. It has been accepted for inclusion in Pediatrics Faculty Publications by an authorized administrator of Health Sciences Research Commons. For more information, please contact [email protected]. Molecular Genetics and Metabolism Reports 3 (2015) 47–54 Contents lists available at ScienceDirect Molecular Genetics and Metabolism Reports journal homepage: http://www.journals.elsevier.com/molecular-genetics-and- metabolism-reports/ Case Report An uncommon clinical presentation of relapsing dilated cardiomyopathy with identification of sequence variations in MYNPC3, KCNH2 and mitochondrial tRNA cysteine Maria J. Guillen Sacoto a,1, Kimberly A. -

Should Genetic Testing Be Recommended for Long QT Syndrome Patients and Their Relatives?
The Science Journal of the Lander College of Arts and Sciences Volume 11 Number 1 Fall 2017 - 2017 Should Genetic Testing Be Recommended for Long QT Syndrome Patients and Their Relatives? Menachem Braun Touro College Follow this and additional works at: https://touroscholar.touro.edu/sjlcas Part of the Cardiovascular Diseases Commons, and the Medical Genetics Commons Recommended Citation Braun, M. (2017). Should Genetic Testing Be Recommended for Long QT Syndrome Patients and Their Relatives?. The Science Journal of the Lander College of Arts and Sciences, 11(1). Retrieved from https://touroscholar.touro.edu/sjlcas/vol11/iss1/8 This Article is brought to you for free and open access by the Lander College of Arts and Sciences at Touro Scholar. It has been accepted for inclusion in The Science Journal of the Lander College of Arts and Sciences by an authorized editor of Touro Scholar. For more information, please contact [email protected]. Should Genetic Testing be Recommended for Long QT Syndrome Patients and Their Relatives? Menachem Braun Menachem Braun graduated with a BS in Biology in September 2017. Abstract 7KH/RQJ476\QGURPH /476 LVDIDPLOLDOSRWHQWLDOO\IDWDOFDUGLDFDUUK\WKPLD7UDGLWLRQDOO\LWKDVEHHQGLDJQRVHGE\(&* Molecular studies have provided evidence that LQTS can be caused by a range of underlying molecular abnormalities. Genetic research has proven that different forms of LQTS have different genotypic bases. Therefore, it has become possible to diagnose WKHVSHFLÀFW\SHRIGLVHDVHJHQHWLFDOO\7KLVVWXG\H[DPLQHVWKHDGYDQFHPHQWVPDGHLQWKHSDVWWKLUW\\HDUVLQXQGHUVWDQGLQJ /476DQGUHVHDUFKUHJDUGLQJWKHXVHRIJHQHWLFWHVWLQJLQRUGHUWRGHWHUPLQHWKHEHQHÀWVRIJHQHWLFWHVWLQJIRUWKLVGLVHDVH$ survey of original studies which produced the information is presented here, and provides the reader with an understanding of the PHFKDQLFVRIWKHGLVHDVHDQGKRZWKH\GLIIHULQWKHVHYHUDOJHQHWLFYDULDQWV5HVHDUFKVKRZVWKDWWKHEHQHÀWRIJHQHWLFWHVWLQJ must be weighed against the personal implications in may have for a particular patient and his or her family. -

Latest Diagnostic and Treatment Strategies for the Congenital Long QT Syndromes
Latest Diagnostic and Treatment Strategies for the Congenital Long QT Syndromes Michael J. Ackerman, MD, PhD Windland Smith Rice Cardiovascular Genomics Research Professor Professor of Medicine, Pediatrics, and Pharmacology Director, Long QT Syndrome Clinic and the Mayo Clinic Windland Smith Rice Sudden Death Genomics Laboratory President, Sudden Arrhythmia Death Syndromes (SADS) Foundation Learning Objectives to Disclose: • To RECOGNIZE the “faces” (phenotypes) of the congenital long QT syndromes (LQTS) • To CRITIQUE the various diagnostic modalities used in the evaluation of LQTS and UNDERSTAND their limitations • To ASSESS the currently available treatment options for the various LQT syndromes and EVALUATE their efficacy WINDLAND Smith Rice Sudden Death Genomics Laboratory Conflicts of Interest to Disclose: • Consultant – Boston Scientific, Gilead Sciences, Medtronic, St. Jude Medical, and Transgenomic/FAMILION • Royalties – Transgenomic/FAMILION Congenital Long QT Syndrome Normal QT interval QT QT Prolonged QT 1. Syncope 2. Seizures 3. Sudden death Torsades de pointes Congenital Long QT Syndrome Normal QT interval QT QT ♥ 1957 – first clinical description – JLNS ♥ 1960s – RomanoProlonged-Ward QT syndrome ♥ 1983 – “Schwartz/Moss score”1. Syncope ♥ 1991 – first LQTS chromosome locus 2. Seizures ♥ March 10, 1995 – birth of cardiac 3. Sudden channelopathies death Torsades de pointes Congenital Long QT Syndrome Normal QT interval QT QT Prolonged QT 1. Syncope 2. Seizures 3. Sudden death Torsades de pointes Congenital Long QT Syndrome Normal -

Long QT Syndrome
orphananesthesia Anaesthesia recommendations for patients suffering from Long QT syndrome Disease name: Long QT syndrome ICD 10: I45.8 Synonyms: LQTS Related syndromes: Romano-Ward S., Jervell and Lange-Nielsen S., Timothy S. Long QT syndrome (LQTS) is a cardiac disorder resulting from malfunction of cardiac ion channels. It can be divided in congenital (c-LQTS) and acquired (a-LQTS) forms. Typical is a prolongation of the QT interval on ECG and a propensity to ventricular tachyarrhythmias which can lead to a characteristic polymorphic ventricular tachycardia known as “torsades de pointes”. There is a risk of syncope, cardiac arrest or sudden death. The diagnosis is based on ECG findings, clinical and family history. The Schwartz score defines the probability of LQTS according to three categories: 1 point, low probability of LQTS; (2) 2 or 3 points, intermediate probability of LQTS; and (3) ≥3.5 points, high probability of LQTS. THE TABLE OF THE SCHWARTZ SCORE should be added here: Table 2 of the paper Schwartz PJ and Crotti L. QTc behavior during exercise and genetic testing for the Long-QT Syndrome: Circulation 2011;124:2181-2184. Medicine in progress Perhaps new knowledge Every patient is unique Perhaps the diagnostic is wrong Find more information on the disease, its centres of reference and patient organisations on Orphanet: www.orpha.net 1 Typical surgery The typical surgery for LQTS is left cardiac sympathetic denervation, which is recommended in patients symptomatic for cardiac events despite beta-blocker (BB) therapy or in patients in whom BBs are contraindicated or not tolerated. For therapeutic reasons the implantation of a cardioverter defibrillator (ICD) is recommended in patients with a previous cardiac arrest or in patients with recurrent syncopal events despite antiadrenergic interventions. -

Update on the Diagnosis and Management of Familial Long QT Syndrome
Heart, Lung and Circulation (2016) 25, 769–776 POSITION STATEMENT 1443-9506/04/$36.00 http://dx.doi.org/10.1016/j.hlc.2016.01.020 Update on the Diagnosis and Management of Familial Long QT Syndrome Kathryn E Waddell-Smith, FRACP a,b, Jonathan R Skinner, FRACP, FCSANZ, FHRS, MD a,b*, members of the CSANZ Genetics Council Writing Group aGreen Lane Paediatric and Congenital Cardiac Services, Starship Children’s Hospital, Auckland New Zealand bDepartment[5_TD$IF] of Paediatrics,[6_TD$IF] Child[7_TD$IF] and[8_TD$IF] Youth[9_TD$IF] Health,[10_TD$IF] University of Auckland, Auckland, New Zealand Received 17 December 2015; accepted 20 January 2016; online published-ahead-of-print 5 March 2016 This update was reviewed by the CSANZ Continuing Education and Recertification Committee and ratified by the CSANZ board in August 2015. Since the CSANZ 2011 guidelines, adjunctive clinical tests have proven useful in the diagnosis of LQTS and are discussed in this update. Understanding of the diagnostic and risk stratifying role of LQTS genetics is also discussed. At least 14 LQTS genes are now thought to be responsible for the disease. High-risk individuals may have multiple mutations, large gene rearrangements, C-loop mutations in KCNQ1, transmembrane mutations in KCNH2, or have certain gene modifiers present, particularly NOS1AP polymorphisms. In regards to treatment, nadolol is preferred, particularly for long QT type 2, and short acting metoprolol should not be used. Thoracoscopic left cardiac sympathectomy is valuable in those who cannot adhere to beta blocker therapy, particularly in long QT type 1. Indications for ICD therapies have been refined; and a primary indication for ICD in post-pubertal females with long QT type 2 and a very long QT interval is emerging. -

Coxsackievirus B Detection in Cases of Myocarditis, Myopericarditis, Pericarditis and Dilated Cardiomyopathy in Hospitalized Patients
MOLECULAR MEDICINE REPORTS 10: 2811-2818, 2014 Coxsackievirus B detection in cases of myocarditis, myopericarditis, pericarditis and dilated cardiomyopathy in hospitalized patients IMED GAALOUL1-3*, SAMIRA RIABI1*, RAFIK HARRATH1, TIMOTHY HUNTER2, KHALDOUN B. HAMDA4, ASSIA B. GHZALA5, SALLY HUBER3 and MAHJOUB AOUNI1 1Laboratory of Transmissible Diseases LR99‑ES27, Faculty of Pharmacy, Monastir 5000, Tunisia; 2DNA Microarray Facility, 305 Health Science Research Facility, University of Vermont; 3Department of Pathology, University of Vermont, Burlington, VT 05405, USA; 4Department of Cardiology, University Hospital Fattouma Bourguiba, Monastir 5000; 5Department of Cardiology, University Hospitals Farhat Hached and Sahloul, Sousse 4054, Tunisia Received November 9, 2013; Accepted May 21, 2014 DOI: 10.3892/mmr.2014.2578 Abstract. Coxsackieviruses B (CV-B) are known as the most Introduction common viral cause of human heart infections. The aim of the present study was to assess the potential role of CV-B in the Cardiovascular infections include a group of entities involving etiology of infectious heart disease in hospitalized patients. the heart wall, such as myocarditis, dilated cardiomyopathy The present study is based on blood, pericardial fluid and heart and pericarditis. These processes are associated with high biopsies from 102 patients and 100 control subjects. All of the morbidity and mortality. Although early diagnosis is essential samples were examined for the detection of specific enteroviral for adequate patient management and leads to improved prog- genome using the reverse transcription polymerase chain reac- nosis, the clinical manifestations are often non specific (1). tion (RT-PCR) and sequence analysis. Immunohistochemical Myocarditis is clinically and pathologically defined as investigations for the detection of the enteroviral capsid an inflammation of the heart muscle.