Ali Hawii 0085A 10685.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

4 Reproductive Biology of Cerambycids
4 Reproductive Biology of Cerambycids Lawrence M. Hanks University of Illinois at Urbana-Champaign Urbana, Illinois Qiao Wang Massey University Palmerston North, New Zealand CONTENTS 4.1 Introduction .................................................................................................................................. 133 4.2 Phenology of Adults ..................................................................................................................... 134 4.3 Diet of Adults ............................................................................................................................... 138 4.4 Location of Host Plants and Mates .............................................................................................. 138 4.5 Recognition of Mates ................................................................................................................... 140 4.6 Copulation .................................................................................................................................... 141 4.7 Larval Host Plants, Oviposition Behavior, and Larval Development .......................................... 142 4.8 Mating Strategy ............................................................................................................................ 144 4.9 Conclusion .................................................................................................................................... 148 Acknowledgments ................................................................................................................................. -

Crop Colonization by Pests and Specialist Enemies
insects Article Dispersal in Host–Parasitoid Interactions: Crop Colonization by Pests and Specialist Enemies Edward W. Evans Department of Biology, Utah State University, Logan, UT 84322-5305, USA; [email protected]; Tel.: +01-435-797-2552 Received: 7 September 2018; Accepted: 2 October 2018; Published: 5 October 2018 Abstract: Interactions of insect pests and their natural enemies increasingly are being considered from a metapopulation perspective, with focus on movements of individuals among habitat patches (e.g., individual crop fields). Biological control may be undercut in short-lived crops as natural enemies lag behind the pests in colonizing newly created habitat. This hypothesis was tested by assessing parasitism of cereal leaf beetle (Oulema melanopus) and alfalfa weevil (Hypera postica) larvae at varying distances along transects into newly planted fields of small grains and alfalfa in northern Utah. The rate of parasitism of cereal leaf beetles and alfalfa weevils by their host-specific parasitoids (Tetrastichus julis (Eulophidae) and Bathyplectes curculionis (Ichneumonidae), respectively) was determined for earliest maturing first generation host larvae. Rates of parasitism did not vary significantly with increasing distance into a newly planted field (up to 250–700 m in individual experiments) from the nearest source field from which pest and parasitoid adults may have immigrated. These results indicate strong, rapid dispersal of the parasitoids in pursuing their prey into new habitat. Thus, across the fragmented agricultural landscape of northern Utah, neither the cereal leaf beetle nor the alfalfa weevil initially gained substantial spatial refuge from parasitism by more strongly dispersing than their natural enemies into newly created habitat. -

Transcriptome and Gene Expression Analysis of Rhynchophorus Ferrugineus (Coleoptera: Curculionidae) During Developmental Stages
Transcriptome and gene expression analysis of Rhynchophorus ferrugineus (Coleoptera: Curculionidae) during developmental stages Hongjun Yang1,2, Danping Xu1, Zhihang Zhuo1,2,3, Jiameng Hu2 and Baoqian Lu4 1 College of Life Science, China West Normal University, Nanchong, Sichuan, China 2 Key Laboratory of Genetics and Germplasm Innovation of Tropical Special Forest Trees and Ornamental Plants, Ministry of Education, Key Laboratory of Germplasm Resources Biology of Tropical Special Ornamental Plants of Hainan Province, College of Forestry, Hainan University, Haikou, Hainan, China 3 Key Laboratory of Integrated Pest Management on Crops in South China, Ministry of Agriculture, South China Agricultural University, Guangzhou, Guangdong, China 4 Key Laboratory of Integrated Pest Management on Tropical Crops, Ministry of Agriculture China, Environ- ment and Plant Protection Institute, Chinese Academy of Tropical Agricultural Sciences, Haikou, Hainan, China ABSTRACT Background. Red palm weevil, Rhynchophorus ferrugineus Olivier, is one of the most destructive pests harming palm trees. However, genomic resources for R. ferrugineus are still lacking, limiting the ability to discover molecular and genetic means of pest control. Methods. In this study, PacBio Iso-Seq and Illumina RNA-seq were used to generate transcriptome from three developmental stages of R. ferrugineus (pupa, 7th-instar larva, adult) to increase the understanding of the life cycle and molecular characteristics of the pest. Results. Sequencing generated 625,983,256 clean reads, from which 63,801 full-length transcripts were assembled with N50 of 3,547 bp. Expression analyses revealed 8,583 differentially expressed genes (DEGs). Moreover, gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis revealed that these Submitted 5 March 2020 Accepted 29 September 2020 DEGs were mainly related to the peroxisome pathway which associated with metabolic Published 2 November 2020 pathways, material transportation and organ tissue formation. -
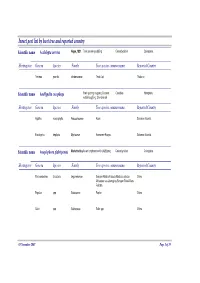
Insect Pest List by Host Tree and Reported Country
Insect pest list by host tree and reported country Scientific name Acalolepta cervina Hope, 1831 Teak canker grub|Eng Cerambycidae Coleoptera Hosting tree Genera Species Family Tree species common name Reported Country Tectona grandis Verbenaceae Teak-Jati Thailand Scientific name Amblypelta cocophaga Fruit spotting bug|eng Coconut Coreidae Hemiptera nutfall bug|Eng, Chinche del Hosting tree Genera Species Family Tree species common name Reported Country Agathis macrophylla Araucariaceae Kauri Solomon Islands Eucalyptus deglupta Myrtaceae Kamarere-Bagras Solomon Islands Scientific name Anoplophora glabripennis Motschulsky Asian longhorn beetle (ALB)|eng Cerambycidae Coleoptera Hosting tree Genera Species Family Tree species common name Reported Country Paraserianthes falcataria Leguminosae Sengon-Albizia-Falcata-Molucca albizia- China Moluccac sau-Jeungjing-Sengon-Batai-Mara- Falcata Populus spp. Salicaceae Poplar China Salix spp. Salicaceae Salix spp. China 05 November 2007 Page 1 of 35 Scientific name Aonidiella orientalis Newstead, Oriental scale|eng Diaspididae Homoptera 1894 Hosting tree Genera Species Family Tree species common name Reported Country Lovoa swynnertonii Meliaceae East African walnut Cameroon Azadirachta indica Meliaceae Melia indica-Neem Nigeria Scientific name Apethymus abdominalis Lepeletier, Tenthredinidae Hymenoptera 1823 Hosting tree Genera Species Family Tree species common name Reported Country Other Coniferous Other Coniferous Romania Scientific name Apriona germari Hope 1831 Long-horned beetle|eng Cerambycidae -

Four New Species of Parasitoid Wasp (Hymenoptera: Braconidae) Described Through a Citizen Science Partnership with Schools in Regional South Australia
Zootaxa 4949 (1): 079–101 ISSN 1175-5326 (print edition) https://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2021 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4949.1.4 http://zoobank.org/urn:lsid:zoobank.org:pub:0C917F76-75A1-4F46-829B-C5143D7AEADA Four new species of parasitoid wasp (Hymenoptera: Braconidae) described through a citizen science partnership with schools in regional South Australia ERINN P. FAGAN-JEFFRIES1,2*, ANDREW D. AUSTIN1,2,4 & CITIZEN SCIENCE PARTICIPANTS OF INSECT INVESTIGATORS3 1Australian Centre for Evolutionary Biology & Biodiversity and School of Biological Sciences, The University of Adelaide, Australia. 2South Australian Museum, Adelaide, Australia 3Students and teachers of Cowell Area School, Macclesfield Primary School, Ramco Primary School and Waikerie Primary School, Australia. 4 [email protected] , https://orcid.org/0000-0002-9602-2276 *Corresponding author. [email protected]; https://orcid.org/0000-0002-3322-6255 Abstract Involving the community in taxonomic research has the potential to increase the awareness, appreciation and value of taxonomy in the public sphere. We report here on a trial citizen science project, Insect Investigators, which partners taxonomists with school students to monitor Malaise traps and prioritise the description of new species collected. In this initial trial, four schools in regional South Australia participated in the program and all collected new species of the braconid subfamily Microgastrinae (Hymenoptera: Braconidae). These four species are here described as new, with the names being chosen in collaboration with the participating school students: Choeras ramcomarmorata Fagan-Jeffries & Austin sp. nov., Glyptapanteles drioplanetus Fagan-Jeffries & Austin sp. -

Hymenoptera: Braconidae) Reared from Hypercompe Cunigunda (Lepidoptera: Erebidae) in Brazil
Revista Brasileira de Entomologia 64(1):e201982, 2020 www.rbentomologia.com Diolcogaster choi sp. nov. from Brazil, a new gregarious microgastrine parasitoid wasp (Hymenoptera: Braconidae) reared from Hypercompe cunigunda (Lepidoptera: Erebidae) in Brazil Geraldo Salgado-Neto1* , Ísis Meri Medri2, José L. Fernández-Triana3, James Bryan Whitfield4 1Universidade Federal de Santa Maria, Departamento de Defesa Fitossanitária, Pós-graduação em Agronomia, Santa Maria, RS, Brasil. 2Universidade de Brasília, Departamento de Ecologia, Doutorado em Ecologia, Brasília, D F, Brasil. 3Canadian National Collection of Insects, Arachnids, and Nematodes, Ottawa, Ontario, Canada. 4University of Illinois at Urbana-Champaign, Department of Entomology, Urbana, USA. urn:lsid:zoobank.org:pub:28F860D2-5CDB-4D55-82BC-C41CFE1ADD0E ARTICLE INFO ABSTRACT Article history: A new species of Diolcogaster (Hymenoptera: Braconidae) is described and illustrated. Additionally, its position Received 23 August 2019 within the recently published key to New World species of the xanthaspis species-group (to which the described Accepted 17 December 2019 Diolcogaster belongs) is provided. The gregarious larval parasitoid Diolcogaster choi sp. nov. was collected in Available online 17 February 2020 Maringá, Paraná State, Brazil. This natural enemy was recovered from a caterpillar of Hypercompe cunigunda (Stoll, Associate Editor: Bernardo Santos 1781) (Lepidoptera: Erebidae) that was feeding on plant of passionflower, Passiflora edulis Sims (Passifloraceae). The fauna of the xanthaspis group in the New World now includes five species, including the new species from Brazil described in this paper. Diolcogaster choi sp. nov. differs anatomically, and is morphologically diagnosed, Keywords: from all other known member of the xanthaspis group of the genus Diolcogaster, to which it belongs. The species Caterpillar also differs in recorded host, and its DNA barcode appears to be distinctive among described Diolcogaster. -

Estimating the Age of the Polydnavirus Braconid Wasp Symbiosis
Estimating the age of the polydnavirus͞braconid wasp symbiosis James B. Whitfield† Department of Entomology, University of Illinois, 320 Morrill Hall, 505 South Goodwin Avenue, Urbana, IL 61801 Edited by May R. Berenbaum, University of Illinois, Urbana, IL, and approved April 1, 2002 (received for review February 4, 2002) Polydnaviruses are essential components mediating host–parasi- between the symbiotic viruses and braconid wasps are reviewed, toid relationships between some braconid wasps and their cater- and estimates are made of the age of this remarkable association pillar hosts largely by suppressing or misdirecting the host immune by using molecular clock-based extrapolations from DNA se- systems. The polydnavirus–wasp relationship is an unusual appar- quence data from three genes, calibrated with dates from wasp ent mutualism between viruses and eukaryotes and remarkably fossils. It is shown that the wasps and viruses are likely to have has evolved to the stage where the two entities no longer can be been associated with one another at least since the Cretaceous- considered separate. Estimations of the age of the polydnavirus- Tertiary boundary and perhaps for even longer. bearing clade of braconid wasps based on separate calculations from the mitochondrial 16S rRNA and COI genes and the nuclear Monophyly of the Polydnavirus-Bearing Clade Within 28S rRNA gene, calibrated using fossil data, converge to indicate a Braconid Wasps date of origin of Ϸ73.7 ؎ 10 million years ago. This range provides an upper bound on the time during which these wasps and viruses The presence of a morphologically distinct class of polydnavi- have been functionally associated. -

Hymenoptera: Braconidae: Microgastrinae) Comb
Revista Brasileira de Entomologia 63 (2019) 238–244 REVISTA BRASILEIRA DE Entomologia A Journal on Insect Diversity and Evolution www.rbentomologia.com Systematics, Morphology and Biogeography First record of Cotesia scotti (Valerio and Whitfield, 2009) (Hymenoptera: Braconidae: Microgastrinae) comb. nov. parasitising Spodoptera cosmioides (Walk, 1858) and Spodoptera eridania (Stoll, 1782) (Lepidoptera: Noctuidae) in Brazil a b a a Josiane Garcia de Freitas , Tamara Akemi Takahashi , Lara L. Figueiredo , Paulo M. Fernandes , c d e Luiza Figueiredo Camargo , Isabela Midori Watanabe , Luís Amilton Foerster , f g,∗ José Fernandez-Triana , Eduardo Mitio Shimbori a Universidade Federal de Goiás, Escola de Agronomia, Setor de Entomologia, Programa de Pós-Graduac¸ ão em Agronomia, Goiânia, GO, Brazil b Universidade Federal do Paraná, Setor de Ciências Agrárias, Programa de Pós-Graduac¸ ão em Agronomia – Produc¸ ão Vegetal, Curitiba, PR, Brazil c Universidade Federal de São Carlos, Programa de Pós-Graduac¸ ão em Ecologia e Recursos Naturais, São Carlos, SP, Brazil d Universidade Federal de São Carlos, Departamento de Ecologia e Biologia Evolutiva, São Carlos, SP, Brazil e Universidade Federal do Paraná, Departamento de Zoologia, Curitiba, PR, Brazil f Canadian National Collection of Insects, Ottawa, Canada g Universidade de São Paulo, Escola Superior de Agricultura “Luiz de Queiroz”, Departamento de Entomologia e Acarologia, Piracicaba, SP, Brazil a b s t r a c t a r t i c l e i n f o Article history: This is the first report of Cotesia scotti (Valerio and Whitfield) comb. nov. in Brazil, attacking larvae of the Received 3 December 2018 black armyworm, Spodoptera cosmioides, and the southern armyworm, S. -

Biological Control of Gonipterus Platensis
BIOLOGICAL CONTROL OF GONIPTERUS PLATENSIS: CURRENT STATUS AND NEW POSSIBILITIES CARLOS MANUEL FERREIRA VALENTE ORIENTADORA: Doutora Manuela Rodrigues Branco Simões TESE ELABORADA PARA OBTENÇÃO DO GRAU DE DOUTOR EM ENGENHARIA FLORESTAL E DOS RECURSOS NATURAIS 2018 BIOLOGICAL CONTROL OF GONIPTERUS PLATENSIS: CURRENT STATUS AND NEW POSSIBILITIES CARLOS MANUEL FERREIRA VALENTE ORIENTADORA: Doutora Manuela Rodrigues Branco Simões TESE ELABORADA PARA OBTENÇÃO DO GRAU DE DOUTOR EM ENGENHARIA FLORESTAL E DOS RECURSOS NATURAIS JÚRI: Presidente: Doutora Maria Teresa Marques Ferreira Professora Catedrática Instituto Superior de Agronomia Universidade de Lisboa Vogais: Doutora Maria Rosa Santos de Paiva Professora Catedrática Faculdade de Ciências e Tecnologia Universidade Nova de Lisboa; Doutora Manuela Rodrigues Branco Simões Professora Auxiliar com Agregação Instituto Superior de Agronomia Universidade de Lisboa; Doutor José Carlos Franco Santos Silva Professor Auxiliar Instituto Superior de Agronomia Universidade de Lisboa; Doutor Edmundo Manuel Rodrigues de Sousa Investigador Auxiliar Instituto Nacional de Investigação Agrária e Veterinária. 2018 À Susana e à Leonor i Em memória da minha Avó, Maria dos Anjos Valente (1927-2017) ii Agradecimentos Agradeço, em primeiro lugar, à Professora Manuela Branco, pelo apoio incansável na orientação desta tese, a total disponibilidade e os inúmeros ensinamentos. Ao RAIZ, pelo financiamento do doutoramento, e à sua Direção, em particular ao Engenheiro Serafim Tavares, ao Engenheiro José Nordeste, ao Professor Carlos Pascoal Neto, à Engenheira Leonor Guedes, ao Gabriel Dehon e ao Nuno Borralho, pelo voto de confiança e incentivo que sempre me transmitiram. Deixo um especial agradecimento à Catarina Gonçalves e à Catarina Afonso, pela amizade, por terem ajudado a manter os projetos do RAIZ e a biofábrica a funcionar, pelas horas infindáveis passadas no laboratório e pelos excelentes contributos científicos que muito melhoraram a qualidade desta tese. -

Tamarixia Radiata Behaviour Is Influenced by Volatiles from Both
insects Article Tamarixia radiata Behaviour is Influenced by Volatiles from Both Plants and Diaphorina citri Nymphs Yan-Mei Liu 1,2 , Shu-Hao Guo 1, Fei-Feng Wang 1, Li-He Zhang 2, Chang-Fei Guo 2, Andrew G. S. Cuthbertson 3, Bao-Li Qiu 1,2 and Wen Sang 1,2,* 1 Key Laboratory of Bio-Pesticide Innovation and Application, Department of Entomology, South China Agricultural University, Guangzhou 510640, China; [email protected] (Y.-M.L.); [email protected] (S.-H.G.); wff[email protected] (F.-F.W.); [email protected] (B.-L.Q.) 2 Engineering Research Center of Biological control, Ministry of Education, Guangzhou 510640, China; [email protected] (L.-H.Z.); [email protected] (C.-F.G.) 3 Independent Science Advisor, York YO41 1LZ, UK; [email protected] * Correspondence: [email protected]; Tel.: +86-20-8528-3717 Received: 7 March 2019; Accepted: 13 May 2019; Published: 16 May 2019 Abstract: Tamarixia radiata (Waterston) is an important ectoparasitoid of the Asian citrus psyllid, Diaphorina citri Kuwayama, a globally destructive pest of citrus. In the present study, a Y-tube olfactometer was employed to investigate whether the parasitoid T. radiata is capable of utilizing the odour source emitted by both plants and insect hosts during its foraging. The odour sources included Murraya paniculata (L.) shoots, 1st, 2nd, 3rd, 4th, and 5th D. citri instar nymphs, both individually and in combinations. Moreover, nymph-stage choice for parasitism, including 3rd, 4th, and 5th D. citri instar nymphs, was carried out. The results indicated that female T. radiata were only significantly attracted to volatiles emitted by M. -

Patterns of Animal Dispersal, Vicariance and Diversification in the Holarctic
Biological Journal of the Linnean Society (2001), 73: 345-390. With 15 figures doi:10.1006/bij1.2001.0542, available online at http;//www.idealibrary.comon IDE bl 0 c Patterns of animal dispersal, vicariance and diversification in the Holarctic ISABEL SANMARTIN1*, HENRIK ENGHOFF' and FREDRIK RONQUISTl 'Department of Systematic Zoology, Evolutionary Biology Centre, Uppsala University, Norbyvugen 180, SE-752 36 Uppsala, Sweden 2Zoologisk Museum, Uniuersitetsparken 15, DK-2100 Copenhagen, Denmark Received 23 October 2000; accepted for publication 25 March 2001 We analysed patterns of animal dispersal, vicariance and diversification in the Holarctic based on complete phylogenies of 57 extant non-marine taxa, together comprising 770 species, documenting biogeographic events from the Late Mesozoic to the present. Four major areas, each corresponding to a historically persistent landmass, were used in the analyses: eastern Nearctic (EN), western Nearctic (WN), eastern Palaeoarctic (EP) and western Palaeoarctic (WP). Parsimony-based tree fitting showed that there is no significantly supported general area cladogram for the dataset. Yet, distributions are strongly phylogenetically conserved, as revealed by dispersal- vicariance analysis (DIVA). DIVA-based permutation tests were used to pinpoint phylogenetically determined biogeographic patterns. Consistent with expectations, continental dispersals (WP-EP and WN-EN) are sig- nificantly more common than palaeocontinental dispersals (WN-EP and EN-WP), which in turn are more common than disjunct dispersals (EN-EP and WN-WP). There is significant dispersal asymmetry both within the Nearctic (WN+EN more common than EN+WN) and the Palaeoarctic (EP+WP more common than WP-tEP). Cross- Beringian faunal connections have traditionally been emphasized but are not more important than cross-Atlantic connections in our data set. -

Nine Newly Recorded Species of the Family Braconidae (Hymenoptera) New to Korea
Anim. Syst. Evol. Divers. Vol. 36, No. 1: 25-30, January 2020 https://doi.org/10.5635/ASED.2020.36.1.043 Review article Nine Newly Recorded Species of the Family Braconidae (Hymenoptera) New to Korea Hye-Rin Lee1,*, S. A. Belokobylskij2, Deok-Seo Ku3, Bong-Kyu Byun4 1Animal Recovery Team (Insects), Division of Restoration Research, National Institute of Ecology, Yeongyang 36531, Korea 2Zoological Institute, Russian Academy of Sciences, St. Petersburg 199034, Russia 3The Science Museum of Natural Enemies, Geochang 50147, Korea 4Department of Biological Science & Biotechnology, Hannam University, Daejeon 34430, Korea ABSTRACT Braconid wasps is second largest family of Hymenoptera. It is comprising more than 18,000 described species. In Korea, a total of 910 species have been recorded to date. In the present study, nine species of the family Braconidae are recorded for the first time from Korea: Aneurobracon philippinensis (Musebeck), Cenocoelius japonicus (Watanabe), Rattana sinica He & Chen, Adelius clandestinus (Förster), Blacometeorus brevicauda (Hellen), Centistes minutus Chen & Achterberg, Centistes sylvicola Belokobylskij, Gnamptodon sichotaealinicus Belokobylskij, Cotesia urabae Austin & Allen. Diagnosis, distribution and host information for the each species are provided. Keywords: Braconidae, Hymenoptera, new record, Korea INTRODUCTION MATERIALS AND METHODS Braconid wasps is second largest family of Hymenoptera, Material examined in this study was deposited at the Science and important insect group in biodiversity. It is comprising Museum of Natural Enemies (SMNE), Geochang, Korea. Ter- more than 18,000 described species (Quicke, 2015), but es- minology used in this paper follows that of Achterberg (1993). timated a total between 42,000 and 43,000 species (Jones et Abbreviations of Korean province used in the present study al., 2009).