Comparison of the Anticonvulsant Potency of Various Diuretic Drugs in the Maximal Electroshock-Induced Seizure Threshold Test in Mice
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Actual Place of Diuretics in Hypertension Treatment
Mini Review J Cardiol & Cardiovasc Ther Volume 3 Issue 4 - March 2017 Copyright © All rights are reserved by Farouk Abcha DOI: 10.19080/JOCCT.2017.03.555616 Actual Place of Diuretics in Hypertension Treatment Farouk Abcha, Marouane Boukhris*, Zied Ibn Elhadj, Lobna Laroussi, Faouzi Addad, Afef Ben Halima and Salem Kachboura Cardiology Department of Abderrahmen Mami Hospital, University of Tunis El Manar, Tunisia Submission: February 03, 2017; Published: March 07, 2017 *Corresponding author: Marouane Boukhris, Cardiology Department of Abderrahmen Mami Hospital, Ariana, Faculty of Medicine of Tunis, University of Tunis El Manar, Tunisia, Tel: ; Email: Abstract Diuretics represent a large and heterogeneous class of drugs, differing from each other by structure, site and mechanism of action. Diuretics are widely used, and have several indications in different cardiovascular disorders, particularly in hypertension and heart failure. Despite the large number of available anti-hypertensive drugs, diuretics remained a cornerstone of hypertension treatment. In the current editorial, we assessed the actual place of different diuretics in the hypertension guidelines focusing on the concept of tailored approach in prescribing them for hypertensive patients. Keywords: Diuretics; Hypertension; Hydrochlorothiazide; Indapamide; Guidelines Introduction Diuretics represent a large and heterogeneous class of drugs, differing from each other by structure, site and mechanism of action. Diuretics are widely used, and have several indications in different cardiovascular disorders, particularly in hypertension and heart failure. Despite the large number of available anti-hypertensive drugs, diuretics remained a cornerstone of hypertension treatment [1]. Indeed, they are the second most commonly prescribed class of antihypertensive medication. For instance, 12% of US adults were prescribed a diuretic, and the relative increase in prescriptions from 1999 through 2012 was 1.4 [2]. -

Approach to Managing Patients with Sulfa Allergy Use of Antibiotic and Nonantibiotic Sulfonamides
CME Approach to managing patients with sulfa allergy Use of antibiotic and nonantibiotic sulfonamides David Ponka, MD, CCFP(EM) ABSTRACT OBJECTIVE To present an approach to use of sulfonamide-based (sulfa) medications for patients with sulfa allergy and to explore whether sulfa medications are contraindicated for patients who require them but are allergic to them. SOURCES OF INFORMATION A search of current pharmacology textbooks and of MEDLINE from 1966 to the present using the MeSH key words “sulfonamide” and “drug sensitivity” revealed review articles, case reports, one observational study (level II evidence), and reports of consensus opinion (level III evidence). MAIN MESSAGE Cross-reactivity between sulfa antibiotics and nonantibiotics is rare, but on occasion it can affect the pharmacologic and clinical management of patients with sulfa allergy. CONCLUSION How a physician approaches using sulfa medications for patients with sulfa allergy depends on the certainty and severity of the initial allergy, on whether alternatives are available, and on whether the contemplated agent belongs to the same category of sulfa medications (ie, antibiotic or nonantibiotic) as the initial offending agent. RÉSUMÉ OBJECTIF Proposer une façon d’utiliser les médicaments à base de sulfamides (sulfas) chez les patients allergiques aux sulfas et vérifier si ces médicaments sont contre-indiqués pour ces patients. SOURCES DE L’INFORMATION Une consultation des récents ouvrages de pharmacologie et de MEDLINE entre 1966 et aujourd’hui à l’aide des mots clés MeSH «sulfonamide» et «drug sensitivity» a permis de repérer plusieurs articles de revue et études de cas, une étude d’observation et des rapports d’opinion consensuelles (preuves de niveau III). -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole -

Diuretics for Hypertension
The Rx Files: Q&A Summary March 1998, LDR DIURETICS IN THE TREATMENT OF HYPERTENSION: CURRENT STATUS 1. When are thiazide diuretics considered first line antihypertensives? Antihypertensive therapy should be individualized by choosing agents that have favorable effects on comorbid conditions. The recent JNC VI report1 recommends thiazides (or â Blockers) as first line agents in the treatment of uncomplicated hypertension except where contraindicated, not tolerated, or concurrent medical conditions favor use of alternate agents (see Appendix 1).1 Thiazide diuretics and â Blockers are the only antihypertensives that have been well documented in long-term controlled trials to reduce cardiovascular morbidity and mortality, particularly MI and stroke.2 Current large-scale trials are underway to determine if other classes of antihypertensives have similar benefits. Thiazides are particularly effective in elderly patients with isolated systolic hypertension.1 They also have favorable effects in patients at risk of osteoporosis. When not used for initial monotherapy, addition of a low dose diuretic as the second agent is recommended unless contraindicated.1 2. Are low dose thiazides as effective in treating hypertension as higher doses? Doses as low as 12.5-25mg of hydrochlorothiazide (HCT), given once daily, have been as effective in lowering blood pressure as higher doses.3 In fact, doses above 25mg have little added benefit but greater incidence of adverse effects.3,7 A dose of 6.25mg HCT can often significantly augment the response of other antihypertensives when used in combination. 3. What about the adverse metabolic effects of thiazides? In doses of 25mg or less, incidence of hypokalemia is <5%, and few patients will actually require potassium supplementation or addition of a K+ sparing diuretic. -

PHRP March 2015
March 2015; Vol. 25(2):e2521518 doi: http://dx.doi.org/10.17061/phrp2521518 www.phrp.com.au Research Manual versus automated coding of free-text self-reported medication data in the 45 and Up Study: a validation study Danijela Gnjidica,b,i, Sallie-Anne Pearsona,c, Sarah N Hilmerb,d, Jim Basilakise, Andrea L Schaffera, Fiona M Blythb,f,g and Emily Banksg,h, on behalf of the High Risk Prescribing Investigators a Faculty of Pharmacy, University of Sydney, NSW, Australia b Sydney Medical School, University of Sydney, NSW, Australia c Sydney School of Public Health, University of Sydney, NSW, Australia d Royal North Shore Hospital and Kolling Institute of Medical Research, Sydney, NSW, Australia e School of Computing, Engineering and Mathematics, University of Western Sydney, NSW, Australia f Centre for Education and Research on Ageing (CERA), Concord Hospital, Sydney, NSW, Australia g The Sax Institute, Sydney, NSW, Australia h National Centre for Epidemiology and Population Health, Australian National University, Canberra, ACT i Corresponding author: [email protected] Article history Abstract Publication date: March 2015 Background: Increasingly, automated methods are being used to code free- Citation: Gnjidic D, Pearson S, Hilmer S, text medication data, but evidence on the validity of these methods is limited. Basilakis J, Schaffer AL, Blyth FM, Banks E. To examine the accuracy of automated coding of previously keyed Manual versus automated coding of free-text Aim: in free-text medication data compared with manual coding of original self-reported medication data in the 45 and Up Study: a validation study. Public Health handwritten free-text responses (the ‘gold standard’). -

Efficacy of Indapamide 1.5 Mg, Sustained Release, in the Lowering of Systolic Blood Pressure
Journal of Human Hypertension (2004) 18, S9–S14 & 2004 Nature Publishing Group All rights reserved 0950-9240/04 $30.00 www.nature.com/jhh ORIGINAL ARTICLE Efficacy of indapamide 1.5 mg, sustained release, in the lowering of systolic blood pressure GM London Service de ne´phrologie, Hoˆpital Manhe`s, Ste Genevie`ve des Bois, France The relationship between the increase in blood pressure indapamide SR resulted in a better or equivalent control and the incidence of cardiovascular disease is well of SBP than treatment with a standard dose of a true recognized today. Studies have shown that more thiazide diuretic (hydrochlorothiazide), a calcium chan- attention should be paid to systolic blood pressure nel blocker (amlodipine), and an angiotensin-converting (SBP) in relation to cardiovascular risk and that enzyme inhibitor (enalapril). No therapeutic escape was therapeutic interventions should preferably focus on observed. All treatments showed good acceptability reducing SBP. The antihypertensive efficacy of indapa- with no unexpected adverse event. In conclusion, mide 1.5 mg sustained release (indapamide SR), a low- indapamide SR is very effective in lowering SBP—a dose thiazide-type diuretic, was assessed on SBP. Three major independent cardiovascular risk factor—notably randomized, double-blind, controlled studies were con- in hypertensive high-risk patients with LVH, the elderly ducted with indapamide SR, over a period of 3 to 12 and diabetics, when compared to major antihyperten- months. Elderly patients or patients with target-organ sive treatments. This SBP-lowering effect is maintained damage, hypertension and left ventricular hypertrophy over the long term. (LVH) (LIVE study) or with type II diabetes with micro- Journal of Human Hypertension (2004) 18, S9–S14. -

INDAPAMIDE 2.5MG TABLETS Indapamide Read All of This Leaflet Carefully Before You Start Taking This Medicine Because It Contains Important Information for You
287.5 x 207 mm - side1 PACKAGE LEAFLET: INFORMATION FOR THE USER INDAPAMIDE 2.5MG TABLETS Indapamide Read all of this leaflet carefully before you start taking this medicine because it contains important information for you. • Keep this leaflet. You may need to read it again • If you have any further questions, ask your doctor or pharmacist • This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours • If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4 • Diuretics or “water tablets”, such as bumetanide, furosemide, thiazides and What is in this leaflet xipamide 1. What Indapamide Tablets are and what they are used for • Diuretics or “water tablets” such as amiloride, spironolactone and triamterene 2. What you need to know before you take Indapamide Tablets which increase the flow of urine without excessive loss of potassium 3. How to take Indapamide Tablets • Lithium, an antidepressant due to the risk of increased level of lithium in the blood 4. Possible side effects • Quinidine or cardioglycosides, such as digoxin, disopyramide, amiodarone 5. How to store Indapamide Tablets and sotalol, ibutilide, dofetilite, digitalis which are used to treat abnormal 6. Contents of the pack and other information heart beat • Lidocaine, flecainide and mexiletine (to treat irregular heart beat) • Intravenous erythromycin, an antibiotic 1. WHAT INDAPAMIDE TABLETS ARE AND WHAT THEY • Pentamidine (used in the treatment of protozoal infections) • Prazosin, which is used to treat high blood pressure ARE USED FOR • Antiepileptics such as oxcarbazepine The name of your medicine is Indapamide 2.5mg Tablets. -

3. Diuretics for Hypertension-A Review and Update
REVIEW Diuretics for Hypertension: A Review and Update George C. Roush1 and Domenic A. Sica2 Downloaded from https://academic.oup.com/ajh/article-abstract/29/10/1130/2622231 by Xenia Agorogianni user on 17 July 2019 This review and update focuses on the clinical features of hydrochlo- ectopy and reduce the risk for sudden cardiac death relative to thi- rothiazide (HCTZ), the thiazide-like agents chlorthalidone (CTDN) and azide-type diuretics used alone. A recent synthesis of 44 trials has indapamide (INDAP), potassium-sparing ENaC inhibitors and aldos- shown that the relative potencies in milligrams among spironolac- terone receptor antagonists, and loop diuretics. Diuretics are the sec- tone (SPIR), amiloride, and eplerenone (EPLER) are approximately ond most commonly prescribed class of antihypertensive medication, from 25 to 10 to 100, respectively, which may be important when SPIR and thiazide-related diuretics have increased at a rate greater than is poorly tolerated. SPIR reduces proteinuria beyond that provided by that of antihypertensive medications as a whole. The latest hyper- other renin angiotensin aldosterone inhibitors. EPLER also reduces tension guidelines have underscored the importance of diuretics for proteinuria and has beneficial effects on endothelial function. While all patients, but particularly for those with salt-sensitive and resist- guidelines often do not differentiate among specific diuretics, this ant hypertension. HCTZ is 4.2–6.2 systolic mm Hg less potent than review demonstrates that these distinctions are important for man- CTDN, angiotensin-converting enzyme inhibitors, beta blockers, and aging hypertension. calcium channel blockers by 24-hour measurements and 5.1 mm Hg systolic less potent than INDAP by office measurements. -
![<[Invented Name]> 4 Mg / 1.25 Mg / 5 Mg Tablets](https://docslib.b-cdn.net/cover/3102/invented-name-4-mg-1-25-mg-5-mg-tablets-1823102.webp)
<[Invented Name]> 4 Mg / 1.25 Mg / 5 Mg Tablets
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT <[Invented name]> 4 mg / 1.25 mg / 5 mg tablets <[Invented name]> 4 mg / 1.25 mg / 10 mg tablets <[Invented name]> 8 mg / 2.5 mg / 5 mg tablets <[Invented name]> 8 mg / 2.5 mg / 10 mg tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION <[Invented name]> 4 mg / 1.25 mg / 5 mg tablets Each tablet contains 4 mg perindopril erbumine equivalent to 3.338 mg perindopril, 1.25 mg indapamide and 5 mg amlodipine as amlodipine besilate. <[Invented name]> 4 mg / 1.25 mg / 10 mg tablets Each tablet contains 4 mg perindopril erbumine equivalent to 3.338 mg perindopril, 1.25 mg indapamide and 10 mg amlodipine as amlodipine besilate. <[Invented name]> 8 mg / 2.5 mg / 5 mg tablets Each tablet contains 8 mg perindopril erbumine equivalent to 6.676 mg perindopril, 2.5 mg indapamide and 5 mg amlodipine as amlodipine besilate. <[Invented name]> 8 mg / 2.5 mg / 10 mg tablets Each tablet contains 8 mg perindopril erbumine equivalent to 6.676 mg perindopril, 2.5 mg indapamide and 10 mg amlodipine as amlodipine besilate. For the full list of excipients, see section 6.1. 3 PHARMACEUTICAL FORM Tablet. <[Invented name]> 4 mg / 1.25 mg / 5 mg tablets: Dark pink marbled rounded tablets of diameter 7 mm embossed "4 1.25 5" on one side. <[Invented name]> 4 mg / 1.25 mg / 10 mg tablets: Pink marbled rounded tablets of diameter 9.4 mm embossed "4 1.25 10" on one side. <[Invented name]> 8 mg / 2.5 mg / 5 mg tablets: Light pink marbled rounded tablets of diameter 9.4 mm embossed "8 2.5 5" on one side. -
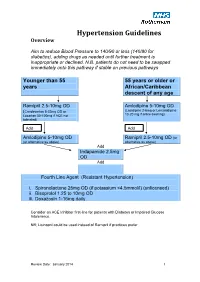
Hypertension Protocol
Hypertension Guidelines Overview Aim to reduce Blood Pressure to 140/90 or less (140/80 for diabetics), adding drugs as needed until further treatment is inappropriate or declined. N.B. patients do not need to be swapped immediately onto this pathway if stable on previous pathways Younger than 55 55 years or older or years African/Caribbean descent of any age Ramipril 2.5-10mg OD Amlodipine 5-10mg OD (Candesartan 8-32mg OD or (Lacidipine 2-6mg or Lercanidipine 10-20 mg if ankle swelling) Losartan 50-100mg if ACE not tolerated) Add Add Amlodipine 5-10mg OD Ramipril 2.5-10mg OD (or (or alternative as above) alternative as above) Add Indapamide 2.5mg OD Add Fourth Line Agent (Resistant Hypertension) i. Spironolactone 25mg OD (if potassium <4.5mmol/l) (unlicensed) ii. Bisoprolol 1.25 to 10mg OD iii. Doxazosin 1-16mg daily Consider an ACE Inhibitor first-line for patients with Diabetes or Impaired Glucose Intolerance. NB; Lisinopril could be used instead of Ramipril if practices prefer Review Date: January 2014 1 Review Date: January 2014 2 General Principles 1. Clinic BP >140/90 leads to Ambulatory Blood Pressure Monitoring (ABPM) 2. ABPM uses average of at least 14 readings taken during waking hours 3. Offer antihypertensive drug to all under 80 with Stage 1 hypertension with: o Target organ damage (high albumin:creatinine ratio, haematuria, raised creatinine, low eGFR, hypertensive retinopathy, LVH) o Established cardiovascular disease o Renal disease o Diabetes o 10 year Cardiovascular risk >20% 4. Offer antihypertensive drug to all with stage 2 hypertension 5. -

Which Diuretics Are Safe and Effective for Patients with a Sulfa Allergy?
From the CLINIcAL InQUiRiES Family Physicians Inquiries Network Ron Healy, MD University of Washington, Which diuretics are safe Seattle; Alaska Family Medicine Residency, Anchorage and effective for patients Terry Ann Jankowski, MLS University of Washington, Seattle with a sulfa allergy? Evidence-based answer Diuretics that do not contain a sulfonamide subsequent allergic reactions to commonly group (eg, amiloride hydrochloride, used sulfonamide-containing diuretics eplerenone, ethacrynic acid, spironolactone, (eg, carbonic anhydrase inhibitors, loop and triamterene) are safe for patients with an diuretics, and thiazides) (strength of allergy to sulfa. The evidence is contradictory recommendation: C, based on case series ® as to whether a history Dowdenof allergy to Healthand poor Media quality case-control and cohort sulfonamide antibiotics increases the risk of studies). Copyright Clinical commentaryFor personal use only Are all sulfa drugs created equal? agents and off-patent, with no company Historical bromides commonly fall by the to take up their cause, no one has been FAST TRACK wayside as better evidence becomes willing to challenge outdated package Reasonable available. Who would have thought 15 insert warnings. years ago that we would be promoting As clinicians who regularly work evidence supports beta-blockers for patients with congestive without a net, we are accustomed to what many of us heart failure? prescribing medications in less than ideal are doing: Using Likewise, with closer inspection, we circumstances. Thankfully, reasonable cheap thiazides have learned that not all sulfa drugs are evidence is available to support what many created equal. The stereospecificity due of us are already doing—using cheap for patients to the absence of aromatic amines in thiazides for patients despite a history of with a history common diuretics means they are safe sulfa allergy. -

Medication Errors
Medication Errors BY MICHAEL R. COHEN, ScD, MS, RPh PRESIDENT OF THE INSTITUTE FOR SAFE MEDICATION PRACTICES LOOK-ALIKE DRUGS spironolactone, which could lead to (Prograf) capsules instead of Astagraf These tablets are hyperkalemia. Or, if indapamide is XL. All tacrolimus products were in mistakenly dispensed instead of the same drop-down menu because almost twins spironolactone, a patient could expe- the hospital’s computer system oth indapamide 2.5 mg (ANI rience hypokalemia from taking two displayed all strengths of an active B Pharmaceuticals) and spironolac- tablets of indapamide. In addition, a ingredient in a single list. Also, tone 25 mg (Amneal Pharmaceuti- patient who uses online tablet/ immediate-release and extended- cals) are round white tablets that are capsule identification resources release tacrolimus products are avail- virtually identical in color, size, and could easily misidentify these tablets. able in similar 0.5 mg, 1 mg, and 5 shape (see photos below). In addi- Pharmacists should ensure that both mg strengths, which may increase tion, indapamide has an imprint brands are not stocked in the pharmacy. the potential for confusion between code of ANI 511 and spironolactone There are alternative manufacturers, the two dosage forms. The error was has a very similar imprint code, AN especially for spironolactone 25 mg. discovered when the patient noticed 511. As shown in the photos, these Technically, these products may a difference in how the capsules qualities make them extremely dif- meet FDA requirements that tablets looked compared with prior refills ficult to tell apart visually, setting the be clearly marked or imprinted with a and reported it to the pharmacy.