For Classical Biological Control of Common Crupina, Crupina Vulgaris (Asteraceae), in the Contiguous United States
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Long-Term Strategy for Russian Olive and Saltcedar Management
LONG-TERM STRATEGY FOR RUSSIAN OLIVE AND SALTCEDAR MANAGEMENT YELLOWSTONE RIVER CONSERVATION DISTRICT COUNCIL Prepared by Thomas L. Pick, Bozeman, Montana May 1, 2013 Cover photo credit: Tom Pick. Left side: Plains cottonwood seedlings on bar following 2011 runoff. Top: Saltcedar and Russian olive infest the shoreline of the Yellowstone River between Hysham and Forsyth, Montana. Bottom: A healthy narrowleaf cottonwood (Populus angustifolia James) stand adjacent to the channel provides benefits for wildlife and livestock in addition to bank stability and storing floodwater for later release. Table of Contents page number Agreement ………………………………………………………………………………………………………………………………………….1 Executive Summary …………………………………………………………………………………………………………………………….2 Long-Term Strategy for Russian Olive and Saltcedar Management 1.0 Introduction ………………………………………………………………………………………………………………………4 1.1 Biology and Ecology of Russian Olive and Saltcedar ……………………………………………………5 1.12 Russian Olive 1.13 Saltcedar 1.2 Distribution and Spread……………………………………………………………………………………………..6 1.3 Summary of Impacts ………………………………………………………………………………………………….6 1.4 Legal Framework ……………………………………………………………………………………………………….7 2.0 Strategic Management Objective and Goals ………………………………………………………………..…..8 2.1 Goal 1: Prevent New Infestations (Control Spread) …………………………………….……….……8 2.2 Goal 2: Eradicate All Infestations Within the River Corridor ………………………………………9 2.3 Goal 3: Manage Populations Outside of the River Corridor ……………………………………….9 3.0 Treatment Strategies and Priorities …………………………………….……………………………………………9 -

The Vascular Plants of Massachusetts
The Vascular Plants of Massachusetts: The Vascular Plants of Massachusetts: A County Checklist • First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Somers Bruce Sorrie and Paul Connolly, Bryan Cullina, Melissa Dow Revision • First A County Checklist Plants of Massachusetts: Vascular The A County Checklist First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Massachusetts Natural Heritage & Endangered Species Program Massachusetts Division of Fisheries and Wildlife Natural Heritage & Endangered Species Program The Natural Heritage & Endangered Species Program (NHESP), part of the Massachusetts Division of Fisheries and Wildlife, is one of the programs forming the Natural Heritage network. NHESP is responsible for the conservation and protection of hundreds of species that are not hunted, fished, trapped, or commercially harvested in the state. The Program's highest priority is protecting the 176 species of vertebrate and invertebrate animals and 259 species of native plants that are officially listed as Endangered, Threatened or of Special Concern in Massachusetts. Endangered species conservation in Massachusetts depends on you! A major source of funding for the protection of rare and endangered species comes from voluntary donations on state income tax forms. Contributions go to the Natural Heritage & Endangered Species Fund, which provides a portion of the operating budget for the Natural Heritage & Endangered Species Program. NHESP protects rare species through biological inventory, -

BIGHEAD KNAPWEED (Centaurea Macrocephala Puschk.)
PNW386 BIGHEAD KNAPWEED (Centaurea macrocephala Puschk.) Bighead knapweed is native to woody crown, each topped by a the mountain grasslands of 3-inch flower head. When Caucasia (specifically Armenia competing with other plants, and Romania) and also to bighead knapweed is generally subalpine meadows in Turkey. only 2 to 3 feet tall with one or European growers cultivate it as two stalks bearing flower heads an ornamental flower and sell it about 1.5 inches in diameter. in the fresh flower markets. Broadly lance-shaped leaves have Although not as popular as sharp-pointed tips, shallowly cornflower (bachelor's button) or toothed edges and rough mountain bluet, it is also surfaces. Blades of the stalked available in the United States in basal and rosette leaves may be flower seed catalogs and 10 inches long and 3 inches wide; nurseries under various common blade and stalk together may names, including Lemon fluff exceed 15 inches. Stem leaves and Globe Centaury. gradually change from having Occasionally, it appears in dried simple stalks to winged stalks to flower arrangements. stalkless as they become smaller Weed specialists in Washington going up the stem. The smallest found bighead knapweed in the Under frroorable conditions, bighead leaves are clustered on the early 1980s escaping from knapweed grows 4 to 5 feet tall. (Each swollen stem at the base of the abandoned gardens in Pend stripe on the stake is 4 inches.) flower head. Oreille and Whitman counties, and added it to the Class A noxious weed list. Since then, Okanogan County, Washington, and Quesnel, British Columbia have reported it. -

Potted Sale Plant MASTER LIST.Xlsx
3/29/2021 Texas Discovery Gardens Plant Sale List Page 1 of 9 ALPHABETICAL BY PLANT GROUP** Sun Req. Tx=Tx Common Name Botanic Name Height Plant Group Plant Type Host / Pollinators native Nectar Attracted & X=Not Comments Hot sun X-Mex Truncate Parry's Agave parryi var. 3 ft Heat & Drought Tolerant Evergreen Agave truncata Hot sun Tx Cholla Cactus Cylindropuntia Heat & Drought Tolerant Evergreen imbricata Hot sun Tx Red Yucca Hesperaloe 4' Heat & Drought Tolerant Evergreen N Hummingbirds parviflora Hot sun Tx Yellow Yucca Hesperaloe 4' X 4' Heat & Drought Tolerant Evergreen N Hummingbirds parviflora yellow Hot sun Tx Hesperaloe Pink Hesperaloe 'Perfu' 4' Heat & Drought Tolerant Evergreen N Hesperaloe funifera cultivar Parade™ x Hesperaloe parviflora Full to part Tx Devil's Shoestring Nolina 3' X 3' Heat & Drought Tolerant Evergreen N sun lindheimeriana Part sun Tx Texas Beargrass Nolina texana Heat & Drought Tolerant Evergreen N/H Sandia Hairstreak Hot sun X- SE US Variegated Yucca gloriosa 4 ft Heat & Drought Tolerant Spanish Dagger 'Variegata' Hot sun Tx Pale Leaf Yucca Yucca pallida 1-2X1-3' Heat & Drought Tolerant Evergreen N Hot sun Tx Twist-leaf Yucca - Yucca rupicola 2 ft Heat & Drought Tolerant Evergreen N Green Hot sun Tx Old Shag Yucca treculeana to 20 ft Heat & Drought Tolerant Evergreen N Don Quixote's- Lace Full to part X Dianthus 'Coral Dianthus 'Coral 1 ft high Heat & Drought Tolerant N sun Reef" Reef" (sun) Hot sun X-Mex Golden Barrel Echinocactus 2 ft Heat & Drought Tolerant Tender Cactus grusonii (sun) Hot sun Tx Prairie Flax Linum lewisii 18 in Heat & Drought Tolerant Perennial (sun) Full sun X-So Am. -

Alhagi Maurorum
Prepared By Jacob Higgs and Tim Higgs Class 1A EDRR- Early Detection Rapid Response Watch List Common crupina Crupina vulgaris African rue Peganum harmala Small bugloss Anchusa arvensis Mediterranean sage Salvia aethiopis Spring millet Milium vernale Syrian beancaper Zygophyllum fabago North Africa grass Ventenata dubia Plumeless thistle Carduus acanthiodes Malta thistle Centaurea melitensis Common Crupina Crupina vulgaris African rue Peganum harmala Small bugloss Anchusa arvensis Mediterranean sage Salvia aethiopis Spring millet Milium vernale Syrian beancaper Zygophyllum fabago North Africa grass Ventenata dubia Plumeless thistle Carduus acanthiodes Malta thistle Centaurea melitensis m Class 1B Early Detection Camelthorn Alhagi maurorum Garlic mustard Alliaria petiolata Purple starthistle Cantaurea calcitrapa Goatsrue Galega officinalis African mustard Brassica tournefortii Giant Reed Arundo donax Japanese Knotweed Polygonum cuspidatum Vipers bugloss Echium vulgare Elongated mustard Brassica elongate Common St. Johnswort Hypericum perforatum L. Oxeye daisy Leucanthemum vulgare Cutleaf vipergrass Scorzonera laciniata Camelthorn Alhagi maurorum Garlic mustard Alliaria petiolata Purple starthistle Cantaurea calcitrapa Goatsrue Galega officinalis African mustard Brassica tournefortii Giant Reed Arundo donax Japanese Knotweed Polygonum cuspidatum Vipers bugloss Echium vulgare Elongated mustard Brassica elongate Common St. Johnswort Hypericum perforatum L. Oxeye daisy Leucanthemum vulgare Cutleaf vipergrass Scorzonera laciniata Class 2 Control -

The Genome of the Emerging Barley Pathogen Ramularia Collo-Cygni Graham R
McGrann et al. BMC Genomics (2016) 17:584 DOI 10.1186/s12864-016-2928-3 RESEARCHARTICLE Open Access The genome of the emerging barley pathogen Ramularia collo-cygni Graham R. D. McGrann1*†, Ambrose Andongabo2†, Elisabet Sjökvist1,4†, Urmi Trivedi5, Francois Dussart1, Maciej Kaczmarek1,6, Ashleigh Mackenzie1, James M. Fountaine1,7, Jeanette M. G. Taylor1, Linda J. Paterson1, Kalina Gorniak1, Fiona Burnett1, Kostya Kanyuka3, Kim E. Hammond-Kosack3, Jason J. Rudd3, Mark Blaxter4,5 and Neil D. Havis1 Abstract Background: Ramularia collo-cygni is a newly important, foliar fungal pathogen of barley that causes the disease Ramularia leaf spot. The fungus exhibits a prolonged endophytic growth stage before switching life habit to become an aggressive, necrotrophic pathogen that causes significant losses to green leaf area and hence grain yield and quality. Results: The R. collo-cygni genome was sequenced using a combination of Illumina and Roche 454 technologies. The draft assembly of 30.3 Mb contained 11,617 predicted gene models. Our phylogenomic analysis confirmed the classification of this ascomycete fungus within the family Mycosphaerellaceae, order Capnodiales of the class Dothideomycetes. A predicted secretome comprising 1053 proteins included redox- related enzymes and carbohydrate-modifying enzymes and proteases. The relative paucity of plant cell wall degrading enzyme genes may be associated with the stealth pathogenesis characteristic of plant pathogens from the Mycosphaerellaceae. A large number of genes associated with secondary metabolite production, including homologs of toxin biosynthesis genes found in other Dothideomycete plant pathogens, were identified. Conclusions: The genome sequence of R. collo-cygni provides a framework for understanding the genetic basis of pathogenesis in this important emerging pathogen. -
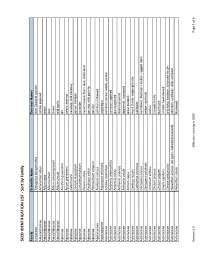
SEED IDENTIFICATION LIST - Sort by Family
SEED IDENTIFICATION LIST - Sort by Family Family Scientific Name Common Names Aizoaceae Tetragonia tetragonoides New Zealand spinach Amaranthaceae Amaranthus albus tumble pigweed Amaryllidaceae Allium cepa onion Amaryllidaceae Allium porrum leek Amaryllidaceae Allium schoenoprasum chives Amaryllidaceae Allium vineale wild garlic Apiaceae Anethum graveolens dill Apiaceae Apium graveolens celery, celeriac Apiaceae Carum carvi caraway; wild caraway Apiaceae Conium maculatum poison hemlock Apiaceae Coriandrum sativum coriander Apiaceae Daucus carota carrot; Queen Ane's lace; wild carrot Apiaceae Pastinaca sativa parsnip; wild parsnip Apiaceae Petroselinum crispum parsley Apocynaceae Asclepias syriaca common milkweed Asparagaceae Asparagus officinalis asparagus Asteraceae Achillea millefolium common yarrow, woolly yarrow Asteraceae Ambrosia artemisiifolia common ragweed Asteraceae Ambrosia trifida giant ragweed Asteraceae Anthemis arvensis field chamomile Asteraceae Anthemis cotula dogfennel, mayweed Asteraceae Arctium lappa great burdock Asteraceae Carduus nutans musk thistle, nodding thistle Asteraceae Carthamus tinctorius safflower Asteraceae Centaurea cyanus cornflower, bachelor's button, ragged robin Asteraceae Centaurea solstitialis yellow starthistle Asteraceae Cichorium endivia endive Asteraceae Cirsium arvense Canada thistle Asteraceae Cirsium vulgare bull thistle Asteraceae Crepis capillaris smooth hawksbeard Asteraceae Cynara cardunculus artichoke, cardoon, artichoke thistle Asteraceae Helianthus annuus (all types, cultivated and -

Ramularia Collo-Cygni Epidemic
bioRxiv preprint doi: https://doi.org/10.1101/215418; this version posted November 7, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. The evolutionary history of the current global Ramularia collo-cygni epidemic Remco Stam1§, Hind Sghyer1*, Martin Münsterkötter4,5*, Saurabh Pophaly2%, Aurélien Tellier2,Ulrich Güldener3, Ralph Hückelhoven1, Michael Hess1§ 1Chair of Phytopathology, 2Section of Population Genetics, 3 Chair of Genome-oriented Bioinformatics Center of Life and Food Sciences Weihenstephan, Technische Universität München, Germany 4Functional Genomics and Bioinformatics, University of Sopron, Hungary 5Institute of Bioinformatics and Systems Biology, Helmholtz Zentrum München, Germany * contributed equally to this work § correspondence: Remco Stam: [email protected] Michael Hess: m.hess@ tum .de % current address: Department of Evolutionary Biology, Evolutionary Biology Centre, Uppsala University, Sweden & Division of Evolutionary Biology, Faculty of Biology II, Ludwig-Maximilians-Universität München, Germany Abstract Ramularia Leaf Spot (RLS) has emerged as a threat for barley production in many regions of the world. Late appearance of unspecific symptoms caused that Ramularia collo-cygni could only by molecular diagnostics be detected as the causal agent of RLS. Although recent research has shed more light on the biology and genomics of the pathogen, the cause of the recent global spread remains unclear. To address urgent questions, especially on the emergence to a major disease, life-cycle, transmission, and quick adaptation to control measures, we de-novo sequenced the genome of R. -

Centaurea Sect
Tesis Doctoral ESTUDIO TAXONÓMICO DE CENTAUREA SECT. SERIDIA (JUSS.) DC. (ASTERACEAE) EN LA PENÍNSULA IBÉRICA E ISLAS BALEARES Memoria presentada por Dña. Vanessa Rodríguez Invernón para optar al grado de Doctor en Ciencias Biológicas por la Universidad de Córdoba Director de Tesis: Prof. Juan Antonio Devesa 15 de octubre de 2013 TITULO: Estudio taxonómico de Centaurea Sect. Seridia (Juss.) DC. en la Península Ibérica e Islas Baleares AUTOR: Vanessa Rodríguez Invernón © Edita: Servicio de Publicaciones de la Universidad de Córdoba. 2013 Campus de Rabanales Ctra. Nacional IV, Km. 396 A 14071 Córdoba www.uco.es/publicaciones [email protected] rírulo DE LA TESIS: Estudio Taxonómico de centaurea sect. seridia (Juss.) DG. en la Península lbérica e Islas Baleares DOCTORANDO/A: VANESSA RODRíGUEZ INVERNÓN INFORME RAZONADO DEL/DE LOS DIRECTOR/ES DE LA TESIS (se hará mención a la evolución y desarrollo de la tesis, así como a trabajos y publicaciones derivados de la misma). El objeto de esta Tesis Doctoral ha sido el estudio taxonómico del género Centaurea, cuya diversidad y complejidad en el territorio es alta, por lo que se ha restringido a la sección Seridia (Juss.) DC. y, atin así, el estudio ha requerido 4 años de dedicación para su finalización. La iniciativa se inscribe en el Proyecto Flora iberica, financiado en la actualidad por el Ministerio de Economía y Competitividad. El estudio ha entrañado la realización de numerosas prospecciones en el campo, necesarias para poder abordar aspectos importantes, tales como los estudios cariológicos, palinológicos y moleculares, todos encaminados a apoyar la slntesis taxonómica, que ha requerido además de un exhaustivo estudio de material conservado en herbarios nacionales e internacionales. -

Nuclear and Plastid DNA Phylogeny of the Tribe Cardueae (Compositae
1 Nuclear and plastid DNA phylogeny of the tribe Cardueae 2 (Compositae) with Hyb-Seq data: A new subtribal classification and a 3 temporal framework for the origin of the tribe and the subtribes 4 5 Sonia Herrando-Morairaa,*, Juan Antonio Callejab, Mercè Galbany-Casalsb, Núria Garcia-Jacasa, Jian- 6 Quan Liuc, Javier López-Alvaradob, Jordi López-Pujola, Jennifer R. Mandeld, Noemí Montes-Morenoa, 7 Cristina Roquetb,e, Llorenç Sáezb, Alexander Sennikovf, Alfonso Susannaa, Roser Vilatersanaa 8 9 a Botanic Institute of Barcelona (IBB, CSIC-ICUB), Pg. del Migdia, s.n., 08038 Barcelona, Spain 10 b Systematics and Evolution of Vascular Plants (UAB) – Associated Unit to CSIC, Departament de 11 Biologia Animal, Biologia Vegetal i Ecologia, Facultat de Biociències, Universitat Autònoma de 12 Barcelona, ES-08193 Bellaterra, Spain 13 c Key Laboratory for Bio-Resources and Eco-Environment, College of Life Sciences, Sichuan University, 14 Chengdu, China 15 d Department of Biological Sciences, University of Memphis, Memphis, TN 38152, USA 16 e Univ. Grenoble Alpes, Univ. Savoie Mont Blanc, CNRS, LECA (Laboratoire d’Ecologie Alpine), FR- 17 38000 Grenoble, France 18 f Botanical Museum, Finnish Museum of Natural History, PO Box 7, FI-00014 University of Helsinki, 19 Finland; and Herbarium, Komarov Botanical Institute of Russian Academy of Sciences, Prof. Popov str. 20 2, 197376 St. Petersburg, Russia 21 22 *Corresponding author at: Botanic Institute of Barcelona (IBB, CSIC-ICUB), Pg. del Migdia, s. n., ES- 23 08038 Barcelona, Spain. E-mail address: [email protected] (S. Herrando-Moraira). 24 25 Abstract 26 Classification of the tribe Cardueae in natural subtribes has always been a challenge due to the lack of 27 support of some critical branches in previous phylogenies based on traditional Sanger markers. -

Taxonomic Studies of Cirsium (Asteraceae) in Japan XXIII. a New Species from Hachiôji, Tokyo Prefecture, Central Japan
Bull. Natl. Mus. Nat. Sci., Ser. B, 38(1), pp. 1–10, February 22, 2012 Taxonomic Studies of Cirsium (Asteraceae) in Japan XXIII. A New Species from Hachiôji, Tokyo Prefecture, Central Japan Yuichi Kadota Department of Botany, National Museum of Nature and Science, Amakubo 4–1–1, Tsukuba, Ibaraki 305–0005, Japan E-mail: [email protected] (Received 14 November 2011; accepted 28 December 2011) Abstract A new species, Cirsium tamastoloniferum Kadota is described from a small marshy land in Hachiôji, Tokyo Pref., central Honshu, Japan, as a member of subsect. Reflexae (the Cirsium kagamontanum group), sect. Onotrophe of the genus Cirsium. Cirsium tamastoloniferum is similar to C. tenuipedunculatum Kadota described from Yamanashi Pref., Chubu District, central Honshu, in having hardly glutinous involucres and paniculate inflorescence with small, numerous heads, however, the former is distinguished from the latter by gynodioecy, subterranean stolons, ovate to broadly ovate cauline leaves with ascending lobes and inner and involucral phyllaries with short- recurved apices in hermaphrodite plants or with short-ascending apices in female plants. Cirsium tamastoloniferum is a dweller of marshy lands exceptionally in the Cirsium kagamontanum group and occurs in Tokyo and Kanagawa Prefs., Kanto District, central Honshu, Japan. Kew words : Cirsium tamastoloniferum, Cirsium tenuipedunculatum, Japan, new species, wet- land. This is part of a revisional work on Japanese Hideshige Uchino, Nagaike Park Nature Center, Cirsium (Asteraceae) (Kadota, 1989–2011; Hachiôji. This thistle seemed to be included in Kadota and Nagase, 1988). In this paper a new the Cirsium kagamontanum group because it had species of subsect. Reflexae (Kitam.) Kadota of paniculate compound inflorescences with small, sect. -

Flora Mediterranea 26
FLORA MEDITERRANEA 26 Published under the auspices of OPTIMA by the Herbarium Mediterraneum Panormitanum Palermo – 2016 FLORA MEDITERRANEA Edited on behalf of the International Foundation pro Herbario Mediterraneo by Francesco M. Raimondo, Werner Greuter & Gianniantonio Domina Editorial board G. Domina (Palermo), F. Garbari (Pisa), W. Greuter (Berlin), S. L. Jury (Reading), G. Kamari (Patras), P. Mazzola (Palermo), S. Pignatti (Roma), F. M. Raimondo (Palermo), C. Salmeri (Palermo), B. Valdés (Sevilla), G. Venturella (Palermo). Advisory Committee P. V. Arrigoni (Firenze) P. Küpfer (Neuchatel) H. M. Burdet (Genève) J. Mathez (Montpellier) A. Carapezza (Palermo) G. Moggi (Firenze) C. D. K. Cook (Zurich) E. Nardi (Firenze) R. Courtecuisse (Lille) P. L. Nimis (Trieste) V. Demoulin (Liège) D. Phitos (Patras) F. Ehrendorfer (Wien) L. Poldini (Trieste) M. Erben (Munchen) R. M. Ros Espín (Murcia) G. Giaccone (Catania) A. Strid (Copenhagen) V. H. Heywood (Reading) B. Zimmer (Berlin) Editorial Office Editorial assistance: A. M. Mannino Editorial secretariat: V. Spadaro & P. Campisi Layout & Tecnical editing: E. Di Gristina & F. La Sorte Design: V. Magro & L. C. Raimondo Redazione di "Flora Mediterranea" Herbarium Mediterraneum Panormitanum, Università di Palermo Via Lincoln, 2 I-90133 Palermo, Italy [email protected] Printed by Luxograph s.r.l., Piazza Bartolomeo da Messina, 2/E - Palermo Registration at Tribunale di Palermo, no. 27 of 12 July 1991 ISSN: 1120-4052 printed, 2240-4538 online DOI: 10.7320/FlMedit26.001 Copyright © by International Foundation pro Herbario Mediterraneo, Palermo Contents V. Hugonnot & L. Chavoutier: A modern record of one of the rarest European mosses, Ptychomitrium incurvum (Ptychomitriaceae), in Eastern Pyrenees, France . 5 P. Chène, M.