Evolutionary Potential in the Alpine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Salix L.) in the European Alps
diversity Review The Evolutionary History, Diversity, and Ecology of Willows (Salix L.) in the European Alps Natascha D. Wagner 1 , Li He 2 and Elvira Hörandl 1,* 1 Department of Systematics, Biodiversity and Evolution of Plants (with Herbarium), University of Goettingen, Untere Karspüle 2, 37073 Göttingen, Germany; [email protected] 2 College of Forestry, Fujian Agriculture and Forestry University, Fuzhou 350002, China; [email protected] * Correspondence: [email protected] Abstract: The genus Salix (willows), with 33 species, represents the most diverse genus of woody plants in the European Alps. Many species dominate subalpine and alpine types of vegetation. Despite a long history of research on willows, the evolutionary and ecological factors for this species richness are poorly known. Here we will review recent progress in research on phylogenetic relation- ships, evolution, ecology, and speciation in alpine willows. Phylogenomic reconstructions suggest multiple colonization of the Alps, probably from the late Miocene onward, and reject hypotheses of a single radiation. Relatives occur in the Arctic and in temperate Eurasia. Most species are widespread in the European mountain systems or in the European lowlands. Within the Alps, species differ eco- logically according to different elevational zones and habitat preferences. Homoploid hybridization is a frequent process in willows and happens mostly after climatic fluctuations and secondary contact. Breakdown of the ecological crossing barriers of species is followed by introgressive hybridization. Polyploidy is an important speciation mechanism, as 40% of species are polyploid, including the four endemic species of the Alps. Phylogenomic data suggest an allopolyploid origin for all taxa analyzed Citation: Wagner, N.D.; He, L.; so far. -

And Betula Nana (Betulaceae)
Acta Societatis Botanicorum Poloniae Journal homepage: pbsociety.org.pl/journals/index.php/asbp ORIGINAL RESEARCH PAPER Received: 2010.09.06 Accepted: 2010.12.29 Published electronically: 2012.10.01 Acta Soc Bot Pol 81(3):153–158 DOI: 10.5586/asbp.2012.027 Calculated characters of leaves are independent on environmental conditions in Salix herbacea (Salicaceae) and Betula nana (Betulaceae) Katarzyna Marcysiak* Department of Botany, Kazimierz Wielki University, Ossolińskich 12, 85–093 Bydgoszcz, Poland Abstract The goal of the study was to check if the shape-describing characters, calculated as ratios of the morphological measured traits are more stable, compared to the latter, and can be treated as independent on environmental conditions. The test was based on the example of leaves of Salix herbacea and Betula nana. The individuals of the two populations ofS. herbacea from Tatra Mts. were divided into two groups: with bigger and smaller leaves. The two populations of B. nana came from different substrata: the first one, collected from the mire on the lower altitude, had bigger leaves, and the second, collected from the granite plateau and higher altitude, had smaller leaves. For both species, the measured traits were generally more variable than the ratios calculated on their basis, as expressed by the variation coefficients. The results of Students' t-test analyses showed statistically significant differences between the two groups of S. herbacea and the two populations of B. nana with respect to almost all the measured characters, and no such differences for the calculated traits, reflecting the leaf shape. As the differentiation of the leaf size was probably bound to the environmental factors, the lack of the dependence of the leaf shape on the leaf size could lead to a conclusion of independence of the leaf shape on the environment conditions. -

Diversity and Evolution of Rosids
*Malpighiales • large and diverse group of 39 families - many of them Diversity and contributing importantly to tropical Evolution of Rosids forest diversity . willows, spurges, and maples . *Salicaceae - willows, poplars *Salicaceae - willows, poplars Chemically defined by salicins (salicylic acid). Many 55 genera, 1000+ species of shrubs/trees - 450 are willows members of the tropical “Flacourtiaceae” with showy flowers (Salix), less numerous are poplars, aspens (Populus). also have salicins and are now part of the Salicaceae Populus deltoides - Salix babylonica - Dovyalis hebecarpa Oncoba spinosa American cottonwood weeping willow 1 *Salicaceae - willows, poplars *Salicaceae - willows, poplars Willows (Salix) are dioecious trees of temperate regions with female male • nectar glands at base of bract allows reduced flowers in aments - both insect and wind pollinated insect as well as wind pollination • fruit is a capsule with cottony seeds for wind dispersal female male Salix babylonica - weeping willow *Salicaceae - willows, poplars *Salicaceae - willows, poplars • species vary from large trees, shrubs, to tiny tundra subshrubs • many species are “precocious” - flower before leaves flush in spring Salix discolor - pussy willow Salix herbacea - Salix pedicellaris - Salix fragilis - dwarf willow bog willow crack willow 2 *Salicaceae - willows, poplars *Salicaceae - willows, poplars Populus - poplars, cottonwood, aspens male • flowers possess a disk • cottony seeds in capsule female Populus deltoides American cottonwood Populus deltoides - American cottonwood *Salicaceae - willows, poplars *Salicaceae - willows, poplars Populus balsamifera Balsam poplar, balm-of-gilead P. tremuloides P. grandidentata trrembling aspen bigtooth aspen • aspens are clonal from root sprouts, fast growing, light Populus alba wooded, and important for White poplar pulp in the paper industry Introduced from Europe 3 *Euphorbiaceae - spurges *Euphorbiaceae - spurges Euphorbiaceae s.l. -

Vascular Flora and Geoecology of Mont De La Table, Gaspésie, Québec
RHODORA, Vol. 117, No. 969, pp. 1–40, 2015 E Copyright 2015 by the New England Botanical Club doi: 10.3119/14-07; first published on-line March 11, 2015. VASCULAR FLORA AND GEOECOLOGY OF MONT DE LA TABLE, GASPE´ SIE, QUE´ BEC SCOTT W. BAILEY USDA Forest Service, 234 Mirror Lake Road, North Woodstock, NH 03262 e-mail: [email protected] JOANN HOY 21 Steam Mill Road, Auburn, NH 03032 CHARLES V. COGBILL 82 Walker Lane, Plainfield, VT 05667 ABSTRACT. The influence of substrate lithology on the distribution of many vascular and nonvascular plants has long been recognized, especially in alpine, subalpine, and other rocky habitats. In particular, plants have been classified as dependent on high-calcium substrates (i.e., calcicoles) based on common restriction to habitats developed in calcareous rocks, such as limestone and marble. In a classic 1907 paper on the influence of substrate on plants, M. L. Fernald singled out a particular meadow on Mont de la Table in the Chic-Choc Mountains of Que´bec for its unusual co-occurrence of strict calcicole and calcifuge (i.e., acidophile) plant taxa. We re-located this site, investigated substrate factors responsible for its unusual plant diversity, and documented current plant distributions. No calcareous rocks were found on site. However, inclusions of calcareous rocks were found farther up the mountain. The highest pH and dissolved calcium concentrations in surface waters were found in a series of springs that deliver groundwater, presumably influenced by calcareous rocks up the slope. Within the habitat delineated by common occurrences of calcicole species, available soil calcium varied by a factor of five and soil pH varied by almost 1.5 units, depending on microtopography and relative connection with groundwater. -

CONSERVATION of ARCTIC FLORA and FAUNA (CAFF) FLORA GROUP Acknowledgements I
CAFF Technical Report No. 15 September 2008 PROCEEDINGS OF THE FOURTH INTERNATIONAL WORKSHOP CONSERVATION OF ARCTIC FLORA AND FAUNA (CAFF) FLORA GROUP Acknowledgements i CAFF Designated Agencies: • Environment Canada, Ottawa, Canada • Finnish Ministry of the Environment, Helsinki, Finland • Ministry of the Environment and Nature, Greenland Homerule, Greenland (Kingdom of Denmark) • Faroese Museum of Natural History, Tórshavn, Faroe Islands (Kingdom of Denmark) • Icelandic Institute of Natural History, Reykjavik, Iceland • Directorate for Nature Management, Trondheim, Norway • Russian Federation Ministry of Natural Resources, Moscow, Russia • Swedish Environmental Protection Agency, Stockholm, Sweden • United States Department of the Interior, Fish and Wildlife Service, Anchorage, Alaska This publication should be cited as: Talbot, S.S., ed. (2008): Proceedings of the Fourth International Conservation of Arctic Flora and Fauna (CAFF) Flora Group Workshop, 15-18 May 2007, Tórshavn, Faroe Islands. CAFF Technical Report No. 15. Akureyri, Iceland. Cover photo: View from Villingadalsfjall (elevation 844m) looking south to Malinsfjall (750m) on the island of Vidoy, Faroe Islands. In the Palaeogene the island was geographically close (100-120 km) to East Greenland. Photo by Anna Maria Fosaa. For more information please contact: CAFF International Secretariat Borgir, Nordurslod 600 Akureyri, Iceland Phone: +354 462-3350 Fax: +354 462-3390 Email: [email protected] Internet: http://www.caff.is ___ CAFF Designated Area Editing: Tammy Charron Design & Layout: Tom Barry Proceedings of the Fourth International Conservation of Arctic Flora and Fauna (CAFF) Flora Group Workshop May 15-18, 2007 Tórshavn, Faroe Islands CAFF Technical Report No. 15 September 2008 Table of Contents 1 Preliminaries Welcome—H. Poulsen . .1 Introduction—S. S. -

Diversity and Evolution of Rosids
Oxalidales • small, heterogeneous, novel group Diversity and of 6 families - seed character? Oxalidaceae Evolution of Rosids Wood sorrels . violets, willows, and spurges . Cephalotaceae Australian pitcher plant Oxalidaceae - wood sorrels Oxalidaceae - wood sorrels 6 genera, 770 species in the tropics and temperate areas - 700 6 genera, 770 species in the tropics and temperate areas - 700 belong to Oxalis (wood sorrel) belong to Oxalis (wood sorrel) • plants are herbaceous creepers or woody Oxalis corniculata - creeping yellow wood sorrel • typically 3-foliate vines leaves (the real shamrock) • leaves are acidic to taste due to oxalic acid in the form of calcium oxalate Oxalidaceae - wood sorrels Oxalidaceae - wood sorrels CA 5 CO 5 A 5+5 G (5) • 5 merous flowers CA 5 CO 5 A 5+5 G (5) • 5 merous flowers Oxalis corniculata Oxalis • fruits are 5 locular & Oxalis corniculata Oxalis • fruits are 5 locular & winged capsules or berries winged capsules or berries • tristyly common (3 levels at which 2 sets of anthers and 1 set of styles position) U U U Oxalidaceae - wood sorrels Oxalidaceae - wood sorrels • common native and introduced wood-sorrels • tropical fruit - carambola or star fruit: note 5 carpellate structure Oxalis stricta - Oxalis violaceae - tall wood-sorrel violet wood-sorrel Averrhoa carambola Oxalis acetosella - wood-sorrel *Malpighiales *Malpighiales • large and diverse group of 38 • unresolved! “novel” clade families - many of them • leaf margin teeth contributing importantly to tropical • “Parietales” subclade (placentation) forest diversity • hosts for Cymothoe butterflies *Malpighiales *Violaceae - violets • unusual life forms 23 genera, 800 species of herbs (temperate) to vines and small trees (tropics). 400-600 of them are violets (Viola). -

Where Do the Treeless Tundra Areas of Northern Highlands
Where do the treeless tundra areas of northern highlands fit in the global biome system: toward an ecologically natural subdivision of the tundra biome Risto Virtanen1, Lauri Oksanen2,3, Tarja Oksanen2,3, Juval Cohen4, Bruce C. Forbes5, Bernt Johansen6, Jukka Kayhk€ o€7, Johan Olofsson8, Jouni Pulliainen4 & Hans Tømmervik9 1Department of Ecology, University of Oulu, FI-90014 Oulu, Finland 2Department of Arctic and Marine Biology, University of Tromsø – The Arctic University of Norway, Campus Alta, NO-9509 Alta, Norway 3Section of Ecology, Department of Biology, University of Turku, FI-20014 Turku, Finland 4Finnish Meteorological Institute, PL 503, 00101 Helsinki, Finland 5Arctic Centre, University of Lapland, P.O. Box 122, FI-96101 Rovaniemi, Finland 6Northern Research Institute, Box 6434, Forskningsparken, NO-9294 Tromsø, Norway 7Department of Geography and Geology, Division of Geography, University of Turku, FI-20014 Turku, Finland 8Department of Ecology and Environmental Science, Umea University, SE-901 87 Umea, Sweden 9The Norwegian Institute for Nature Research (NINA), Framsenteret, NO-9296 Tromsø, Norway Keywords Abstract Alpine, arctic, biome delimitation, ecoregion, mountains, tundra ecosystems, vegetation According to some treatises, arctic and alpine sub-biomes are ecologically simi- pattern, winter climate. lar, whereas others find them highly dissimilar. Most peculiarly, large areas of northern tundra highlands fall outside of the two recent subdivisions of the tun- Correspondence dra biome. We seek an ecologically natural resolution -

Small-Scale Plant Species Distribution in Snowbeds and Its Sensitivity to Climate Change
Plant Ecol DOI 10.1007/s11258-008-9435-9 Small-scale plant species distribution in snowbeds and its sensitivity to climate change Christian Scho¨b Æ Peter M. Kammer Æ Philippe Choler Æ Heinz Veit Received: 12 November 2007 / Accepted: 29 April 2008 Ó Springer Science+Business Media B.V. 2008 Abstract Alpine snowbeds are characterized by a environmental gradients towards later snowmelt date long-lasting snow cover and low soil temperature or lower daily maximum temperature. Furthermore, during the growing season. Both these key abiotic the occurrence pattern of the species along the factors controlling plant life in snowbeds are sensitive snowmelt gradient allowed the establishment of five to anthropogenic climate change and will alter the species categories with different predictions of their environmental conditions in snowbeds to a consider- distribution in a warmer world. The dominants able extent until the end of this century. In order to increased their relative cover with later snowmelt name winners and losers of climate change among date and will, therefore, lose abundance due to the plant species inhabiting snowbeds, we analyzed climate change, but resist complete disappearance the small-scale species distribution along the snow- from the snowbeds. The indifferents and the tran- melt and soil temperature gradients within alpine sients increased in species number and relative cover snowbeds in the Swiss Alps. The results show that the with higher temperature and will profit from climate date of snowmelt and soil temperature were relevant warming. The snowbed specialists will be the most abiotic factors for small-scale vegetation patterns suffering species due to the loss of their habitats as a within alpine snowbed communities. -
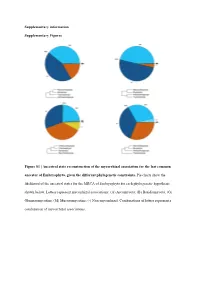
Ancestral State Reconstruction of the Mycorrhizal Association for the Last Common Ancestor of Embryophyta, Given the Different Phylogenetic Constraints
Supplementary information Supplementary Figures Figure S1 | Ancestral state reconstruction of the mycorrhizal association for the last common ancestor of Embryophyta, given the different phylogenetic constraints. Pie charts show the likelihood of the ancestral states for the MRCA of Embryophyta for each phylogenetic hypothesis shown below. Letters represent mycorrhizal associations: (A) Ascomycota; (B) Basidiomycota; (G) Glomeromycotina; (M) Mucoromycotina; (-) Non-mycorrhizal. Combinations of letters represent a combination of mycorrhizal associations. Austrocedrus chilensis Chamaecyparis obtusa Sequoiadendron giganteum Prumnopitys taxifolia Prumnopitys Prumnopitys montana Prumnopitys Prumnopitys ferruginea Prumnopitys Araucaria angustifolia Araucaria Dacrycarpus dacrydioides Dacrycarpus Taxus baccata Podocarpus oleifolius Podocarpus Afrocarpus falcatus Afrocarpus Ephedra fragilis Nymphaea alba Nymphaea Gnetum gnemon Abies alba Abies balsamea Austrobaileya scandens Austrobaileya Abies nordmanniana Thalictrum minus Thalictrum Abies homolepis Caltha palustris Caltha Abies magnifica ia repens Ranunculus Abies religiosa Ranunculus montanus Ranunculus Clematis vitalba Clematis Keteleeria davidiana Anemone patens Anemone Tsuga canadensis Vitis vinifera Vitis Tsuga mertensiana Saxifraga oppositifolia Saxifraga Larix decidua Hypericum maculatum Hypericum Larix gmelinii Phyllanthus calycinus Phyllanthus Larix kaempferi Hieronyma oblonga Hieronyma Pseudotsuga menziesii Salix reinii Salix Picea abies Salix polaris Salix Picea crassifolia Salix herbacea -

Phylogenetic Distribution and Evolution of Mycorrhizas in Land Plants
Mycorrhiza (2006) 16: 299–363 DOI 10.1007/s00572-005-0033-6 REVIEW B. Wang . Y.-L. Qiu Phylogenetic distribution and evolution of mycorrhizas in land plants Received: 22 June 2005 / Accepted: 15 December 2005 / Published online: 6 May 2006 # Springer-Verlag 2006 Abstract A survey of 659 papers mostly published since plants (Pirozynski and Malloch 1975; Malloch et al. 1980; 1987 was conducted to compile a checklist of mycorrhizal Harley and Harley 1987; Trappe 1987; Selosse and Le Tacon occurrence among 3,617 species (263 families) of land 1998;Readetal.2000; Brundrett 2002). Since Nägeli first plants. A plant phylogeny was then used to map the my- described them in 1842 (see Koide and Mosse 2004), only a corrhizal information to examine evolutionary patterns. Sev- few major surveys have been conducted on their phyloge- eral findings from this survey enhance our understanding of netic distribution in various groups of land plants either by the roles of mycorrhizas in the origin and subsequent diver- retrieving information from literature or through direct ob- sification of land plants. First, 80 and 92% of surveyed land servation (Trappe 1987; Harley and Harley 1987;Newman plant species and families are mycorrhizal. Second, arbus- and Reddell 1987). Trappe (1987) gathered information on cular mycorrhiza (AM) is the predominant and ancestral type the presence and absence of mycorrhizas in 6,507 species of of mycorrhiza in land plants. Its occurrence in a vast majority angiosperms investigated in previous studies and mapped the of land plants and early-diverging lineages of liverworts phylogenetic distribution of mycorrhizas using the classifi- suggests that the origin of AM probably coincided with the cation system by Cronquist (1981). -

Ecoregions of North America ''' ·After the Classification of J
SCALE 1:12 000 000 300 0 300 600 MILES E===c===~~C=========~E=====~===3 ECOREGIONS OF NORTH AMERICA ''' ·AFTER THE CLASSIFICATION OF J. M. CROWLEY By Robert G. Bailey, U.S. Forest Service Assisted by Charles T. Cushwa, U.S. Fish and Wildlife Service 1981 ECOREGIONS The ecoregions outlined on this map represent ecos!,rstems of regional extent. Regions are distinguished.according to the Crow ley classification, based on their distinctive climates, vegetation, and sons. The boundaries and numeric codes are modified and refined from ecoregion maps of North America (Crowley, unpub lished), Canada (Crowley 1967), and the United States (Baney 1976). The complete ecoregion code is a three-digit number that ·identifies the three ecologic levelg..-.;.d,omc..in, division, and province-into which the continent has been divided for the purpose of fish and wildlife analysis and data management. This scheme is one of many that geographers have proposed to break down the complex ecological mosaic into simple patterns. Note that it is highly generalized; sharp local differences occur, notably in highland areas. These areas are shown on the map by letter codes and overprint symbols. The domains are subcontinental areas of broad climatic simi larity, such as lands having the dry climates of Koppen (Tre wartha 1943). The divisions, which are subdivisions of the domains, are determined by isolating areas of differing vegetation and regional climates, generally at the level of the basic climatic types of KOppen. Usually the zonal soils are related. The provinces correspond to broad vegetation regions having a uniform regional climate and the same type or types of zonal solls. -

Salix Herbacea L. (Salicaceae) in the Maramures Massif of the Ukrainian Carpathians
Plant Introduction, 85/86, 130–136 (2020) https://doi.org/10.46341/PI2020010 UDC 582.623.2 (477.87) SHORT COMMUNICATION Salix herbacea L. (Salicaceae) in the Maramures massif of the Ukrainian Carpathians R. Hleb 1, 2 *, V. Loya 2, R. Cherepanyn 3 1 Carpathian Biosphere Reserve, Krasne Pleso str. 77, 90600 Rakhiv, Ukraine; * [email protected] 2 M.M. Gryshko National Botanical Garden, National Academy of Sciences of Ukraine, Timiryazevska str. 1, 01014 Kyiv, Ukraine 3 Vasyl Stefanyk Precarpathian National University, Shevchenko str. 57, 76018 Ivano-Frankivsk, Ukraine Received: 19.02.2020 | Accepted: 09.05.2020 | Published: 30.06.2020 Abstract Salix herbacea is a relict plant species related to the circumpolar arctic-alpine element of the Holarctic group. The aim of the study was to clarify the data on the distribution of S. herbacea within the Maramures massif of the Ukrainian Carpathians since this species is reported by different authors for the massif without specific geographical and habitats descriptions. Field studies were conducted in the Maramures massif on the slopes of Pip Ivan Marmarosky (1936 m a.s.l.) and Rapa (1872 m a.s.l.) mountains in 2017– 2019. The S. herbacea distribution chorology was analyzed based on the inventory of UU, KW, KWHA, LW, Herbarium of the Carpathian Biosphere Reserve, and Herbarium of the Biology and Ecology Department of the Vasyl Stefanyk Precarpathian National University collections. We have found S. herbacea confined to cliffs on a rocky tourist path between the peaks of Pip Ivan Marmarosky and Rapa mountains in the Rakhiv district of the Transcarpathian region (Zakarpattia oblast).