OBSERVATIONS Range 11–325), Ruling out a Condition of References Iron Overload
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Footprints an Informational Newsletter for Patients of APMA Member Podiatrists October 2017
footprints an informational newsletter for patients of APMA member podiatrists october 2017 special EDITION include a DPM for diabetes prevention & Manage Ment According to the CDC, more than 100 million US adults are living with either diabetes or prediabetes. If you have diabetes or prediabetes, it is essential to include a podiatrist for proper diabetes prevention and management. In fact, podiatrists can reduce amputation rates up to 80 percent. In order for you not to become a part of that statistic, here are a few simple things you can do to keep your risk for diabetic ulcers and amputation low: Inspect feet daily. Check your Don’t go barefoot. Don’t go 1 feet and toes every day for 5 without shoes, even in your cuts, bruises, sores, or changes to own home. The risk of cuts and the toenails, such as thickening or infection is too great for those discoloration. with diabetes. Wear thick, soft socks. Avoid Never try to remove calluses, 2 socks with seams, which could 6 corns, or warts by yourself. rub and cause blisters or other Over-the-counter products can burn skin injuries. the skin and cause irreparable damage to the foot for people Exercise. Walking can keep with diabetes. 3 weight down and improve circulation. Be sure to wear See a podiatrist. Regular appropriate athletic shoes when 7 checkups by a podiatrist— exercising. at least annually—are the best WALKING CAN KEEP way to ensure that your feet WEIGHT DOWN AND Have new shoes properly remain healthy. 4 measured and fitted. Foot size IMPROVE CIRCULATION. -
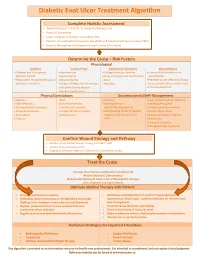
Diabetic Foot Ulcer Treatment Algorithm
Diabetic Foot Ulcer Treatment Algorithm Complete Holistic Assessment Medical/Surgical History & Co-morbidity Management Physical Examination Lower Leg (LLA) including monofilament test Perform Arterial Brachial Pressure Index (ABPI) and Toe Brachial Pressure Index (TBPI) Diabetic Management (Glycemic and Lipid Control & Nutrition) Determine the Cause – Risk Factors Physiological Diabetes Vascular Flow Autoimmune Disorders Wound History Diabetes and sub-optimal Hypertension Collagen vascular diseases History of foot infections or glycemic control Heart disease Immunosuppressant medications osteomyelitis Neuropathic changes with lack of Hyperlipidemia Gout Presence of toe infections (fungal protective sensation History of deep vein thrombosis Vasculitis or bacterial), callous and/or corns Peripheral artery disease Previous ulceration Venous Insufficiency Physical Limitations Socioeconomic/Self-Management Obesity Gait Smoking Lack of awareness for self-care Foot deformity Nutritional deficits Inadequate foot Inadequate hygiene Decreased level of activity Limited joint mobility wear/offloading devices Unsafe home environment Visual disturbances Congenital abnormalities Lack/Inability to afford diabetic Alcohol/drug abuse Amputation Osteoporosis supplies, foot care and foot Decreased Cognitive Ability Trauma wear Depression Financial insecurity Decreased level of activity Confirm Wound Etiology and Pathway Results of LLA, Monofilament Testing and ABPI/ TBPI Results of wound assessment Diagnostic -

Associations Between Nutrients and Foot Ulceration in Diabetes: a Systematic Review
nutrients Review Associations between Nutrients and Foot Ulceration in Diabetes: A Systematic Review Nada Bechara 1,2,3, Jenny E. Gunton 2,3,4,* , Victoria Flood 5,6 , Tien-Ming Hng 1 and Clare McGloin 1 1 Department of Diabetes and Endocrinology, Blacktown-Mt Druitt Hospital, Blacktown, NSW 2148, Australia; [email protected] (N.B.); [email protected] (T.-M.H.); [email protected] (C.M.) 2 Westmead Hospital, Sydney Medical School, Faculty of Medicine and Health, The University of Sydney, Westmead, NSW 2145, Australia 3 Centre for Diabetes, Obesity and Endocrinology Research (CDOER), The Westmead Institute for Medical Research, The University of Sydney, Westmead, NSW 2145, Australia 4 Garvan Institute of Medical Research, Darlinghurst, NSW 2010, Australia 5 Westmead Hospital, Research and Education Network, Western Sydney Local Health District, Westmead, NSW 2145, Australia; [email protected] 6 Faculty of Medicine and Health, Sydney School of Health Sciences, The University of Sydney, Camperdown, NSW 2006, Australia * Correspondence: [email protected] Abstract: We reviewed the literature to evaluate potential associations between vitamins, nutrients, nutritional status or nutritional interventions and presence or healing of foot ulceration in diabetes. Embase, Medline, PubMed, and the Cochrane Library were searched for studies published prior to September 2020. We assessed eligible studies for the association between nutritional status or interventions and foot ulcers. Fifteen studies met the inclusion criteria and were included in this Citation: Bechara, N.; Gunton, J.E.; review. Overall, there is a correlation between poor nutritional status and the presence of foot Flood, V.; Hng, T.-M.; McGloin, C. -

Diabetic Ulcer Identification & Treatment
Diabetic Ulcer Identification & Treatment Bill Richlen PT, WCC, DWC, Clinical Instructor Wound Care Education Institute The information in this handout reflects the state of knowledge, current at time of publication; however, the authors do not take responsibility for data, information, and significant findings related to the topics discussed that became known to the general public following publication. The recommendations contained herein may not be appropriate for use in all circumstances. Decisions to adopt any particular recommendation must be made by the practitioner in light of available resources and circumstances presented by individual patients. Acknowledgements: This handout and presentation was prepared using information generally acknowledged to be consistent with current industry standards. The authors whose works are cited in the Bibliography Section of this manual are hereby recognized and appreciated. All product names, logos and trademarks used in this presentation are the property of the respective trademark owners. ® and ™ denote registered trademarks in the United States and other countries. "The use of NPUAP/EPUAP/PPPIA material does not imply endorsement of products or programs associated with the use of the material." Wound Care Education Institute (WCEI) Phone: 877-462-9234 Email: [email protected] www.wcei.net Copyright © 2018 by Wound Care Education Institute All Rights Reserved. WCEI grants permission for photocopying for limited personal or educational use only. This consent does not extend to other kinds of copying, such as copying for general distribution, for advertising or promotional purposes, for creating new collective works, or for resale. Treating Chronic Diabetic Wounds Outline I. Chronic Wounds A. Some Details 1. Definition: A wound that has failed to proceed orderly through the healing process or fails to result in durable closure.3 a. -

Clinician Assessment Tools for Patients with Diabetic Foot Disease: a Systematic Review
Journal of Clinical Medicine Review Clinician Assessment Tools for Patients with Diabetic Foot Disease: A Systematic Review Raúl Fernández-Torres 1 , María Ruiz-Muñoz 1,* , Alberto J. Pérez-Panero 1 , Jerónimo C. García-Romero 2 and Manuel Gónzalez-Sánchez 3 1 Department of Nursing and Podiatry, University of Málaga, Arquitecto Francisco Peñalosa, s/n. Ampliación Campus de Teatinos, 29071 Málaga, Spain; [email protected] (R.F.-T.); [email protected] (A.J.P.-P.) 2 Medical School of Physical Education and Sports, University of Málaga, C/Jiménez Fraud 10. Edificio López de Peñalver, 29010 Málaga, Spain; [email protected] 3 Department of Physiotherapy, University of Málaga, Arquitecto Francisco Peñalosa, s/n. Ampliación campus de Teatinos, 29071 Málaga, Spain; [email protected] * Correspondence: [email protected]; Tel.: +34-951953215 Received: 10 April 2020; Accepted: 12 May 2020; Published: 15 May 2020 Abstract: The amputation rate in patients with diabetes is 15 to 40 times higher than in patients without diabetes. To avoid major complications, the identification of high-risk in patients with diabetes through early assessment highlights as a crucial action. Clinician assessment tools are scales in which clinical examiners are specifically trained to make a correct judgment based on patient outcomes that helps to identify at-risk patients and monitor the intervention. The aim of this study is to carry out a systematic review of valid and reliable Clinician assessment tools for measuring diabetic foot disease-related variables and analysing their psychometric properties. The databases used were PubMed, Scopus, SciELO, CINAHL, Cochrane, PEDro, and EMBASE. The search terms used were foot, ankle, diabetes, diabetic foot, assessment, tools, instruments, score, scale, validity, and reliability. -

Dynamic Approaches to Improve Glycemic Control and Primary Diabetes Care 1‑Or 2‑Or 3‑Or
DYNAMIC APPROACHES TO IMPROVE GLYCEMIC CONTROL AND PRIMARY DIABETES CARE DYNAMIC APPROACHES TO IMPROVE GLYCEMIC Figure. CONTROL AND PRIMARY DIABETES CARE 1‑OR Characterizing Clinical Inertia in a Large, National Database CORI R. RATTELMAN, ANUPAMA ARORA, JOHN K. CUDDEBACK, ELIZABETH L. CIEMINS, Alexandria, VA Objective: To characterize clinical inertia in the treatment of diabetes using a large, geographically diverse clinical database. Study Design: A retrospective descriptive analysis was conducted in a clinical database containing 22 million patient records across 22 health care organizations (HCOs). Population Studied: A total of 281,000 patients aged 18-75 were included during the 5.5-year study period (1/2012-6/2017). Patients had an outpatient visit in the last 12 months of the study period, an HbA1c in the last 24-30 months (index A1c), and a diagnosis of type 2 DM on a claim or EHR prob- lem list at least 6 months prior to index A1c. A subset of 47,693 patients with an index A1c ≥8 and a prior A1c ≥8 or lack thereof, was observed for Supported By: National Institute of Diabetes and Digestive and Kidney Diseases four 6-month follow-up periods for actions including a new class of diabetes (T35DK104689); Yale University medication prescribed or an A1c <8. The absence of observable action fol- lowing index A1c suggests potential “clinical inertia.” Principal Findings: Six months following an index A1c≥8, 55% of patients 3‑OR received no observable clinical action ranging from 45-65% across HCOs Influence of Diabetes Complications on the Cost‑Effectiveness of and 18-96% across individual providers. -

Study the Effect of Zinc/Or Vit.D3 on Percentage of Healing Of
www.ijapbc.com IJAPBC – Vol. 2(4), Oct-Dec, 2013 ISSN: 2277 - 4688 _________________________________________________________________________________ INTERNATIONAL JOURNAL OF ADVANCES IN PHARMACY, BIOLOGY AND CHEMISTRY Research Article Study the effect of Zinc/or Vit.D3 on Percentage of Healing of Diabetic Foot Ulcer in Iraqi Patients Noor Muhammed A Rahman1, Kassim Al-Shamma,a2 and Salah Kadhim Al-Ahmady1 1Department of Clinical Pharmacy, Pharmacy College, Basrah University, Basrah, Iraq. 2 Department of Clinical Pharmacy, Pharmacy College, Baghdad University, Baghdad, Iraq. ABSTRACT To compare the effect of zinc and vit.D3 on fructosamine level, percentage of healing, and lipid profile in diabetic patient who suffered from diabetic foot ulcer. This study is carried out on 45 diabetic patients suffered from diabetic foot ulcer(s) and 12 healthy subjects. Patients were divided into three groups and assigned for treatment with zinc gluconate (15) patients, vit.D3 (15) patients and placebo group (15) patients for 4weeks. We observed fructosamine level, lipid profile and healing percentage before and after 4 weeks of treatment. There is significantly decrease in fructosamine level in medication treated group after 4 weeks of treatment. There is no significant changing in High density lipoprotein, and Low density lipoprote. Keywords: diabetic foot ulcer, fructosamine, percentage of healing, zinc, vit.D3. INTRODUCTION be complicated by infection and gangrene, leading Diabetes is a group of metabolic disease to long term in hospital treatment and \or characterized by hyperglycemia resulting from amputation8. In recent decades, the knowledge on defects in insulin secretion, insulin action, or both. DFU has clearly increased, with a rise in the The chronic hyperglycemia of diabetes is number of scientific publications and the associated with long-term damage, dysfunction, production of guidelines on prevention and and failure of different organs, especially the eyes, management9. -

Diabetic Foot Ulcer Severity Predicts Mortality Among Veterans with Type 2 Diabetes
Journal of Diabetes and Its Complications xxx (2016) xxx–xxx Contents lists available at ScienceDirect Journal of Diabetes and Its Complications journal homepage: WWW.JDCJOURNAL.COM Diabetic foot ulcer severity predicts mortality among veterans with type 2 diabetes Meghan B. Brennan a,b,c,⁎, Timothy M. Hess a,b, Brian Bartle c, Jennifer M. Cooper d, Jonathan Kang b, Elbert S. Huang c,d, Maureen Smith a, Min-Woong Sohn c,e, Christopher Crnich a,b a University of Wisconsin School of Medicine and Public Health, 1685 Highland Ave, Madison, WI 53705 b William S. Middleton Memorial Veterans Hospital, 2500 Overlook Terrace, Madison, WI 53705 c Edward Hines Jr. Veterans Hospital, 5000 S 5th Ave, Hines, IL 60141 d University of Chicago Medical Center, 5841 S Maryland Ave, Chicago, IL 60637 e University of Virginia School of Medicine, 1215 Lee St, Charlottesville, VA 22908 article info abstract Article history: Aim: Diabetic foot ulcers are associated with an increased risk of death. We evaluated whether ulcer severity Received 29 July 2016 at presentation predicts mortality. Received in revised form 7 November 2016 Methods: Patients from a national, retrospective, cohort of veterans with type 2 diabetes who developed Accepted 28 November 2016 incident diabetic foot ulcers between January 1, 2006 and September 1, 2010, were followed until death or the Available online xxxx end of the study period, January 1, 2012. Ulcers were characterized as early stage, osteomyelitis, or gangrene at presentation. Cox proportional hazard regression identified independent predictors of death, controlling Keywords: Diabetes for comorbidities, laboratory parameters, and healthcare utilization. Foot ulcer Results: 66,323 veterans were included in the cohort and followed for a mean of 27.7 months: 1-, 2-, and 5-year Gangrene survival rates were 80.80%, 69.01% and 28.64%, respectively. -

Role of Hemoglobin A1c As Predictor of Foot Ulcer Healing in Diabetes
Print ISSN: 2321-6379 Online ISSN: 2395-1893 Original Article DOI: 10.17354/SUR/2018/149 Role of Hemoglobin A1c as Predictor of Foot Ulcer Healing in Diabetes H R Manjunath1, Vasudev Murthy Kumar2 1Assistant Professor, Department of General Surgery, Sapthagiri Institute of Medical Sciences and Research Centre, Bengaluru, Karnataka, India, 2Associate Professor, Department of General Surgery, Akash Institute of Medical Sciences and Research Centre, Bengaluru, Karnataka, India Abstract Background: Diabetic foot ulcers are associated with substantial morbidity and mortality in diabetic patients. The role of hemoglobin A1c (HbA1c) in assessing the outcome of diabetic ulcer needs to be evaluated. Aim: This study aims to assess the validity and effectiveness of HbA1c to predict foot ulcer healing in diabetic patients. Materials and Methods: This study was conducted in the Department of Surgery, Akash Institute of Medical Sciences and Research Centre, Devanahalli, over 24-month period from February 2015 to February 2017. A descriptive study was carried out in 280 diabetic patients with foot ulcers. The data collection included demographics, medical diagnoses, ulcer healing duration, and HbA1c results. Results: About 50% of patients with controlled HbA1c levels had their wounds healed in 3 months, whereas only 20% of patients with high HbA1c had their wounds healed in same time. 15% of patients with high HbA1c had their wounds persisting even after 1 year. Conclusion: HbA1c levels were strongly associated with the process as well as the duration of foot ulcer healing in the diabetic patients. Elevated HbA1c was associated with poor prognosis in terms of foot ulcer healing. Key words: Diabetic foot ulcers, Hemoglobin A1c, Morbidity, Mortality INTRODUCTION The factors that affect ulcer healing in diabetic patients are generally helpful in optimization of patient management ypically, diabetic foot syndrome is characterized by strategy besides their routine application as predictors Tfoot infection, ulceration, or destruction of deep of the outcome. -

Diabetic Foot Or Pressure Ulcer on the Foot?
WOUNDS UK DEBATE Diabetic foot or pressure ulcer on the foot? Diabetes is one of the greatest health A pressure ulcer is defined as an The National Institute for Health challenges facing the United Kingdom area of localised damage to the skin and Clinical Excellence provide (UK) today, with 2.5 million people caused by prolonged or excessive soft guidance for practitioners on best diagnosed with diabetes in the UK, tissue pressure along with shear or practice treatment on individuals not including up to half a million friction, or a combination of these with either a diabetic foot ulcer people who have the condition but (European Pressure Ulcer Advisory (NICE, 2004) or a pressure ulcer are unaware of it (Diabetes UK, 2009). Panel [EPUAP], 2005). (NICE, 2005). However, how can we be satisfied that appropriate Disease of the foot is a The heel has been identified guidance is being followed by the complication of diabetes caused by as the second most common site multidisciplinary team? Diabetes UK damage to the nerves and blood for pressure ulcer development, strongly recommend that effective vessels that serve the limbs (Diabetes accounting for up to 28% of all management of disease of the foot in UK, 2009). Active foot disease may be reported ulcers (Barczak et al, 1997). diabetes, requires effective integration either of recent onset or chronic Indeed, Clark et al (2004) concurred, of the input of different healthcare but deteriorating. identifying in their survey that 261 professionals, who together have the (25.8%) patients experienced their skills necessary to assess and treat The term active foot disease refers most severe ulcers in the heel area. -

Type 2 Diabetes Mellitus with Plantar Malignant Melanoma: Report of Two Cases and Literature Review
Type 2 Diabetes Mellitus with Plantar Malignant Melanoma: Report of Two Cases and Literature Review Bi-ling Huang Central South University First Hospital: Xiangya Hospital Central South University Min Tan Central South University First Hospital: Xiangya Hospital Central South University Jie-Yu Liang Central South University First Hospital: Xiangya Hospital Central South University Ming-Liu Li Central South University First Hospital: Xiangya Hospital Central South University Lan Liao Central South University First Hospital: Xiangya Hospital Central South University Min Zhou Central South University First Hospital: Xiangya Hospital Central South University Min Wang ( [email protected] ) Central South University First Hospital: Xiangya Hospital Central South University https://orcid.org/0000-0003-2111- 3074 Case Report Keywords: Type 2 diabetes, diabetic foot ulcer, malignant melanoma, case report. Posted Date: March 15th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-318639/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/10 Abstract Background: Malignant melanoma is a highly malignant tumour that originates from melanocytes. Its prognosis is poor and motality rate is high. Malignant melanoma usually occurs in the skin, also known as cutaneous melanoma,but rarely in the foot. Case report: Here we report two cases of type 2 diabetes diagnosed as malignant melanoma of the foot and provides a review of literature. The rst patient was a 70-year-old woman with a 7 year history of diabetes, who had a ulcer in the right heel for half a year. The second case, which occurred in a 66-year-old woman whose third toe of the right foot had been red, swollen and blackened for 2 months. -

IWGDF Guidelines on the Prevention and Management of Diabetic Foot Disease
2019 IWGDF Guidelines on the prevention and management of diabetic foot disease Practical 6 Guideline Development and Guidelines Chapters methodology IWGDF Guidelines IWGDF EDITORIAL BOARD Nicolaas C. Schaper (chair), Jaap J. van Netten (secretary), Jan Apelqvist, Sicco A. Bus, Robert J. Hinchlife, Benjamin A. Lipsky CORRESPONDENCE www.iwgdfguidelines.org/contact LAYOUT Simon Christiaanse www.simonchristiaanse.com www.iwgdfguidelines.org IWGDF Practical guidelines on the prevention and management of diabetic foot disease Part of the 2019 IWGDF Guidelines on the Prevention and Management of Diabetic Foot Disease IWGDF Guidelines AUTHORS Nicolaas C. Schaper1, Jaap J. van Netten2,3,4, Jan Apelqvist5, Sicco A. Bus2, Robert J. Hinchlife6, Benjamin A. Lipsky7 on behalf of the International Working Group on the Diabetic Foot (IWGDF) INSTITUTIONS 1 Div. Endocrinology, MUMC+, CARIM and CAPHRI Institute, Maastricht, The Netherlands 2 Amsterdam UMC, Department of Rehabilitation Medicine, Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands 3 School of Clinical Sciences, Queensland University of Technology, Brisbane, Australia 4 Diabetic foot clinic, Department of Surgery, Ziekenhuisgroep Twente, Almelo and Hengelo, The Netherlands 5 Department of Endocrinology, University Hospital of Malmö, Sweden 6 Bristol Centre for Surgical Research, University of Bristol, Bristol, UK 7 Department of Medicine, University of Washington, Seattle, USA; Green Templeton College, University of Oxford, Oxford, UK KEYWORDS diabetic foot; foot ulcer; guidelines; guidance; implementation; prevention; treatment WWW.iwgdfguidelines.org IWGDF Practical Guidelines ABSTRACT Diabetic foot disease results in a major global burden for patients and the health care system. The International working Group on the Diabetic Foot (IWGDF) has been producing evidence-based guidelines on the prevention and management of diabetic foot disease since 1999.