Clinician Assessment Tools for Patients with Diabetic Foot Disease: a Systematic Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Arterial Leg Ulcer Clinical Pathway
Waterloo Wellington Integrated Wound Care Program Evidence-Based Wound Care Arterial Leg Ulcer Clinical Pathway 0-7 Days Expected Outcomes Notes Most Responsible Physician(MRP)/Nurse Practitioner (NP) Refer patient to ‘Care Connects’ if no responsible practitioner currently involved with patient identified/informed Determine if MRP/NP is part of Family Health Team (FHT) or Community Health Centre (CHC) and consider additional supports available Medical/surgical history and co-morbidity management Risk factors include: Chronic renal disease considered within care plan Smoking Congestive heart failure Diabetes mellitus Impaired liver function Hyperlipidemia Use of systemic steroids, Hypertension immunosuppressive and chemotherapy Coronary artery disease >70 years of age History of cerebral vascular accident Age 50-69 years with history of diabetes (CVA) or smoking Low hemoglobin < 50 years with diabetes and one other Obesity atherosclerotic factor Poor nutrition History of vascular surgery or deep vein Decreased thyroid function thrombosis Psoriasis Bleeding disorders Autoimmune diseases Family history of arterial disease Medication reconciliation and their impact on wound healing Prescription, non-prescription, naturopathic and illicit drug use (including e-cigarettes, reviewed inhaled substances and nicotine replacement therapy) Medications that can affect healing include: chemotherapy, anticoagulants, antiplatelets, corticosteroids, vasoconstrictors, antihypertensives, diuretics and immunosepressive drugs Other -

Footprints an Informational Newsletter for Patients of APMA Member Podiatrists October 2017
footprints an informational newsletter for patients of APMA member podiatrists october 2017 special EDITION include a DPM for diabetes prevention & Manage Ment According to the CDC, more than 100 million US adults are living with either diabetes or prediabetes. If you have diabetes or prediabetes, it is essential to include a podiatrist for proper diabetes prevention and management. In fact, podiatrists can reduce amputation rates up to 80 percent. In order for you not to become a part of that statistic, here are a few simple things you can do to keep your risk for diabetic ulcers and amputation low: Inspect feet daily. Check your Don’t go barefoot. Don’t go 1 feet and toes every day for 5 without shoes, even in your cuts, bruises, sores, or changes to own home. The risk of cuts and the toenails, such as thickening or infection is too great for those discoloration. with diabetes. Wear thick, soft socks. Avoid Never try to remove calluses, 2 socks with seams, which could 6 corns, or warts by yourself. rub and cause blisters or other Over-the-counter products can burn skin injuries. the skin and cause irreparable damage to the foot for people Exercise. Walking can keep with diabetes. 3 weight down and improve circulation. Be sure to wear See a podiatrist. Regular appropriate athletic shoes when 7 checkups by a podiatrist— exercising. at least annually—are the best WALKING CAN KEEP way to ensure that your feet WEIGHT DOWN AND Have new shoes properly remain healthy. 4 measured and fitted. Foot size IMPROVE CIRCULATION. -

Diabetic Foot Ulcers
REVIEW Review Diabetic foot ulcers William J Jeffcoate, Keith G Harding Ulceration of the foot in diabetes is common and disabling and frequently leads to amputation of the leg. Mortality is high and healed ulcers often recur. The pathogenesis of foot ulceration is complex, clinical presentation variable, and management requires early expert assessment. Interventions should be directed at infection, peripheral ischaemia, and abnormal pressure loading caused by peripheral neuropathy and limited joint mobility. Despite treatment, ulcers readily become chronic wounds. Diabetic foot ulcers have been neglected in health-care research and planning, and clinical practice is based more on opinion than scientific fact. Furthermore, the pathological processes are poorly understood and poorly taught and communication between the many specialties involved is disjointed and insensitive to the needs of patients. In this review, we describe the epidemiology, pathogenesis, operations above the ankle or those proximal to the and management of diabetic foot ulceration, and its effect tarsometatarsal joint, and a multinational WHO survey on on patients and society. The condition deserves more lower extremity amputation also included cases of attention, both from those who provide care and those who unoperated gangrene.13 fund research. Moreover, amputation is a marker not just of disease but also of disease management. The decision to operate Epidemiology is determined by many factors, which vary between Incidence and prevalence centres and patients. A high amputation rate might result Although accurate figures are difficult to obtain for the from high disease prevalence, late presentation, and prevalence or incidence of foot ulcers, the results of cross- inadequate resources, but could also reflect a particular sectional community surveys in the UK showed that 5·3% approach by local surgeons. -
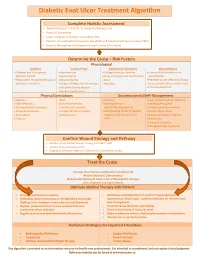
Diabetic Foot Ulcer Treatment Algorithm
Diabetic Foot Ulcer Treatment Algorithm Complete Holistic Assessment Medical/Surgical History & Co-morbidity Management Physical Examination Lower Leg (LLA) including monofilament test Perform Arterial Brachial Pressure Index (ABPI) and Toe Brachial Pressure Index (TBPI) Diabetic Management (Glycemic and Lipid Control & Nutrition) Determine the Cause – Risk Factors Physiological Diabetes Vascular Flow Autoimmune Disorders Wound History Diabetes and sub-optimal Hypertension Collagen vascular diseases History of foot infections or glycemic control Heart disease Immunosuppressant medications osteomyelitis Neuropathic changes with lack of Hyperlipidemia Gout Presence of toe infections (fungal protective sensation History of deep vein thrombosis Vasculitis or bacterial), callous and/or corns Peripheral artery disease Previous ulceration Venous Insufficiency Physical Limitations Socioeconomic/Self-Management Obesity Gait Smoking Lack of awareness for self-care Foot deformity Nutritional deficits Inadequate foot Inadequate hygiene Decreased level of activity Limited joint mobility wear/offloading devices Unsafe home environment Visual disturbances Congenital abnormalities Lack/Inability to afford diabetic Alcohol/drug abuse Amputation Osteoporosis supplies, foot care and foot Decreased Cognitive Ability Trauma wear Depression Financial insecurity Decreased level of activity Confirm Wound Etiology and Pathway Results of LLA, Monofilament Testing and ABPI/ TBPI Results of wound assessment Diagnostic -

The Triangle of Wound Assessment a Simple and Holistic Framework for Wound Management
The Triangle of Wound Assessment A simple and holistic framework for wound management Wound bed WOUND Wound edge Periwound skin We asked healthcare professionals around the world about their priorities ? for wound care We found that most people Respondents said that treating wounds are not protecting the periwound specialists in a hospital1 skin is very important1 Approximately Up to 79% of wounds are 70% of being treated in wounds are the community2 surrounded by unhealthy skin3 2 ...none However, in a recent met all of the study of 14 wound criteria for assessment tools ... optimal wound assessment4 The Triangle of Wound Assessment is a holistic framework that allows practitioners üto assess and manage all areas of the wound, including the periwound skin. It is a simple and systematic approach that guides the user Wound bed from complete wound WOUND assessment to setting management goals, and Wound edge Periwound skin selecting the optimal treatment. 3 The Triangle of Wound Assessment offers a systematic approach to wound management Optimal wound management starts with a holistic wound assessment. This will help to more efficiently set management goals, which will increase the potential for better treatment outcomes. Assessment Management Goals Treatment 4 This is achieved through a holistic framework The Triangle of Wound Assessment provides a framework to assess all three areas of the wound while remembering the patient behind the wound within their social context. Patient Wound bed Social context WOUND Wound Wound edge Periwound skin 5 It’s not just about the wound but also the patient behind the wound Optimal management of the wound starts with assessing the patient behind the wound, and the social context in which the patient lives. -

Guideline: Wound Bed Preparation for Healable and Non Healable Wounds
British Columbia Provincial Nursing Skin and Wound Committee Guideline: Wound Bed Preparation for Healable and Non Healable Wounds Developed by the BC Provincial Nursing Skin and Wound Committee in collaboration with Wound Clinicians from: / TITLE Guideline: Wound Bed Preparation for Healable and Non-Healable Wounds in Adults & Children1 Practice Level Nurses in accordance with health authority and agency policy. Conservative sharp wound debridement (CSWD) is a restricted activity according to the Nurse’s (Registered) and Nurse Practitioner Regulation. 2 CRNBC states that registered nurses must successfully complete additional education and follow an established guideline when carrying out CSWD. Biological debridement therapy is a restricted activity according to the Nurse’s (Registered) and Nurse Practitioner Regulation. 3 CRNBC states that registered nurses must follow an established guideline when carrying out biological debridement. Clients 4 with wounds needing wound bed preparation require an interprofessional approach to provide comprehensive, evidence-based assessment and treatment. This clinical practice guideline focuses solely on the role of the nurse, as one member of the interprofessional team providing care to these clients. Background Factors affecting wound healability include the presence of adequate circulation in the area of the wound, wound related factors such as the size and duration of the wound, the ability to treat the cause of the wound and the presence of risk factors impacting wound healing. While many wounds heal, others are determined to be non-healing or slow-to-heal based on the presence or absence of these factors. Wound healability must be determined prior to debridement and moist wound healing. Although wound healing normally occurs in a predictable fashion, wound healing trajectories can be heterogeneous and non- uniform resulting is delayed wound healing for some clients. -

Associations Between Nutrients and Foot Ulceration in Diabetes: a Systematic Review
nutrients Review Associations between Nutrients and Foot Ulceration in Diabetes: A Systematic Review Nada Bechara 1,2,3, Jenny E. Gunton 2,3,4,* , Victoria Flood 5,6 , Tien-Ming Hng 1 and Clare McGloin 1 1 Department of Diabetes and Endocrinology, Blacktown-Mt Druitt Hospital, Blacktown, NSW 2148, Australia; [email protected] (N.B.); [email protected] (T.-M.H.); [email protected] (C.M.) 2 Westmead Hospital, Sydney Medical School, Faculty of Medicine and Health, The University of Sydney, Westmead, NSW 2145, Australia 3 Centre for Diabetes, Obesity and Endocrinology Research (CDOER), The Westmead Institute for Medical Research, The University of Sydney, Westmead, NSW 2145, Australia 4 Garvan Institute of Medical Research, Darlinghurst, NSW 2010, Australia 5 Westmead Hospital, Research and Education Network, Western Sydney Local Health District, Westmead, NSW 2145, Australia; [email protected] 6 Faculty of Medicine and Health, Sydney School of Health Sciences, The University of Sydney, Camperdown, NSW 2006, Australia * Correspondence: [email protected] Abstract: We reviewed the literature to evaluate potential associations between vitamins, nutrients, nutritional status or nutritional interventions and presence or healing of foot ulceration in diabetes. Embase, Medline, PubMed, and the Cochrane Library were searched for studies published prior to September 2020. We assessed eligible studies for the association between nutritional status or interventions and foot ulcers. Fifteen studies met the inclusion criteria and were included in this Citation: Bechara, N.; Gunton, J.E.; review. Overall, there is a correlation between poor nutritional status and the presence of foot Flood, V.; Hng, T.-M.; McGloin, C. -

Data Entry II Training Course: Supervisor
Training Data Entry II Training Course: Supervisor PCC Supervisor/Manager PCC Operation Guidelines Rating for one Operator Annual Production Rating Formula Fair 20,000 visits 85 forms per day x 230 days per year Good 40,000 visits 170 forms per day x 230 days per year Outstanding 60,000 visits 260 forms per day x 230 days per year 2 PCC Data Entry Needs and Qualifications • On-Reference Material including: • English & Medical Dictionaries • Medical Abbreviations Book • Up-to-date ICD Coding Books • Drug Handbook-Nursing • Data Entry of 20,000 Forms per year by Trained Individual (about 80 forms per day using six to eight mnemonics). • Number of forms processed or data items entered dependent upon each Area or Site. 3 PCC Data Entry Needs and Qualifications (cont.) • Data Entry Staff with the following skills: • Computer keyboard skills • Ability to read provider’s handwriting • Knowledge and understanding of Medical Terminology • Knowledge and understanding of ICD Coding • Knowledge and understanding of Anatomy and Physiology 4 The International Classification of Diseases • ICD-9-CM: 9th Revision, Clinical Modification • The RPMS Coding System for Diagnoses and Procedures 5 ICD Lookup Process • Enter Provider Narrative • FQ Used File • Synonym File • Key Word File • Possible Code(s) 6 Diabetes Diagnosis Codes 250.00 - 250.93 • Type 1 Diabetes - Insulin Dependent Diabetes (Fairly Rare) • Use the Following fifth digit: • 1 - Indicates controlled • 3 - Indicates uncontrolled Note: Requires insulin to survive 7 Diabetes Diagnosis Codes 250.00 - 250.93 (cont.) • Type 2 Diabetes - Non-Insulin Dependent Diabetes (Most frequent Type) • Use the Following fifth-digit: • 0 - Indicates controlled • 2- Indicates uncontrolled Note: Not dependent on insulin to survive; can produce sufficient amounts but may receive insulin to assist the body in using the insulin present in the body. -

Dosing Pattern of Insulin in Diabetic Foot Ulcer Patients TPI 2019; 8(6): 1196-1199 © 2019 TPI B Sarah Fazeeha, P Nivetha, Albin Eldhose, Dr
The Pharma Innovation Journal 2019; 8(6): 1196-1199 ISSN (E): 2277- 7695 ISSN (P): 2349-8242 NAAS Rating: 5.03 Dosing pattern of insulin in diabetic foot ulcer patients TPI 2019; 8(6): 1196-1199 © 2019 TPI www.thepharmajournal.com B Sarah Fazeeha, P Nivetha, Albin Eldhose, Dr. G Veeramani and Dr. J Received: 04-04-2019 Accepted: 06-05-2019 Kabalimurthy B Sarah Fazeeha Abstract Department of Pharmacy, Background: Among the Diabetic patients, 15% develop a foot ulcer and 12-24% of patients with Annamalai University, Chidambaram, Tamil Nadu, diabetic foot ulcer require amputation. We compared the effectiveness of various dosing pattern of India insulin statistically and planned to observe wound healing by proper glycemic control. Methods: We compared the effectiveness of sliding and fixed scale dosing pattern of insulin and P Nivetha observed the wound healing by proper glycemic control. Total 50 subjects were recruited with Grade 3, 4 Department of Pharmacy, and 5 Diabetic foot ulcer (Wagner’s classification) between 40 and 70 years of age who underwent Annamalai University, surgery for management of Foot ulcer. The study was conducted in Tertiary care teaching hospital in Chidambaram, Tamil Nadu, Chidambaram. The study period was 6 month and it was a prospective observational study. The blood India glucose level (Fasting, Random and Post prandial) of subjects were monitored on daily basis and observed. Albin Eldhose Results: The study reports suggested that the major risk factors for Diabetic Foot Ulcer were Poor Department of Pharmacy, glycemic control, Male gender, left foot, Ulcer of size more than 2cm, Infection. -

Diabetic Neuropathy: a Position Statement by the American
136 Diabetes Care Volume 40, January 2017 Diabetic Neuropathy: A Position Rodica Pop-Busui,1 Andrew J.M. Boulton,2 Eva L. Feldman,3 Vera Bril,4 Roy Freeman,5 Statement by the American Rayaz A. Malik,6 Jay M. Sosenko,7 and Dan Ziegler8 Diabetes Association Diabetes Care 2017;40:136–154 | DOI: 10.2337/dc16-2042 Diabetic neuropathies are the most prevalent chronic complications of diabetes. This heterogeneous group of conditions affects different parts of the nervous system and presents with diverse clinical manifestations. The early recognition and appropriate man- agement of neuropathy in the patient with diabetes is important for a number of reasons: 1. Diabetic neuropathy is a diagnosis of exclusion. Nondiabetic neuropathies may be present in patients with diabetes and may be treatable by specific measures. 2. A number of treatment options exist for symptomatic diabetic neuropathy. 3. Up to 50% of diabetic peripheral neuropathies may be asymptomatic. If not recognized and if preventive foot care is not implemented, patients are at risk for injuries to their insensate feet. 4. Recognition and treatment of autonomic neuropathy may improve symptoms, POSITION STATEMENT reduce sequelae, and improve quality of life. Among the various forms of diabetic neuropathy, distal symmetric polyneurop- athy (DSPN) and diabetic autonomic neuropathies, particularly cardiovascular au- tonomic neuropathy (CAN), are by far the most studied (1–4). There are several 1 atypical forms of diabetic neuropathy as well (1–4). Patients with prediabetes may Division of Metabolism, Endocrinology & Diabe- – tes, Department of Internal Medicine, University also develop neuropathies that are similar to diabetic neuropathies (5 10). -

11. Microvascular Complications and Foot Care: Standards of Medical
Diabetes Care Volume 43, Supplement 1, January 2020 S135 11. Microvascular Complications American Diabetes Association and Foot Care: Standards of Medical Care in Diabetes22020 Diabetes Care 2020;43(Suppl. 1):S135–S151 | https://doi.org/10.2337/dc20-s011 11. MICROVASCULAR COMPLICATIONS AND FOOT CARE The American Diabetes Association (ADA) “Standards of Medical Care in Diabetes” includes the ADA’s current clinical practice recommendations and is intended to provide the components of diabetes care, general treatment goals and guidelines, and tools to evaluate quality of care. Members of the ADA Professional Practice Committee, a multidisciplinary expert committee (https://doi.org/10.2337/dc20- SPPC), are responsible for updating the Standards of Care annually, or more frequently as warranted. For a detailed description of ADA standards, statements, and reports, as well as the evidence-grading system for ADA’s clinical practice recommendations, please refer to the Standards of Care Introduction (https://doi.org/10.2337/dc20-SINT). Readers who wish to comment on the Standards of Care are invited to do so at professional.diabetes.org/SOC. For prevention and management of diabetes complications in children and adoles- cents, please refer to Section 13 “Children and Adolescents” (https://doi.org/10.2337/ dc20-S013). CHRONIC KIDNEY DISEASE Screening Recommendations 11.1 At least once a year, assess urinary albumin (e.g., spot urinary albumin-to- creatinine ratio) and estimated glomerular filtration rate (eGFR) in patients with type 1 diabetes with duration of $5 years and in all patients with type 2 diabetes regardless of treatment. B Patients with urinary albumin .30 mg/g creatinine and/or an eGFR ,60 mL/min/1.73 m2 should be monitored twice annually to guide therapy. -

A Focus on the Triangle of Wound Assessment — Addressing the Gap Challenge and Identifying Suspected Biofilm in Clinical Practice
Clinical practice A focus on the Triangle of Wound Assessment — addressing the gap challenge and identifying suspected biofilm in clinical practice Authors: Wound assessment should be comprehensive, systematic and evidence- Caroline Dowsett, Terry Swanson and Tonny Karlsmark based (World Union of Wound Healing Societies [WUWHS], 2016a). The Triangle of Wound Assessment offers clinicians a framework to assess the patient and their wound, taking into consideration the wound bed, wound edge and periwound skin (Dowsett et al, 2015). The framework can be adapted to incorporate new developments and new challenges in wound care such as the ‘gap challenge’ and biofilm prevention and management. Using the framework can assist in determining the status of the wound bed and support clinical decision making to prevent problems associated with exudate pooling at the wound bed and the potential for biofilm formation. he Triangle of Wound Assessment This article will discuss how the Triangle of was established in 2014 and provides Wound Assessment identifies infection and T a systematic approach to wound biofilm, tackles the gap challenge, and how assessment and in setting management goals, this framework can be developed for new to guide optimal treatment choice (Dowsett et challenges in wound care. al, 2015), ensuring that the periwound skin is incorporated into the assessment. Periwound The importance of holistic assessment skin can be a significant problem in patients Wounds are a significant source of cost to with chronic wounds, with between 60–70% patients, as well as to the health economy. of wounds found to be surrounded by either Chronic wounds are often hard to heal problematic or unhealthy periwound skin resulting in a cycle of pain, anxiety and (Cartier et al, 2014).