Prevention and Management of Diabetic Foot Ulcers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clinical Practice Guideline for Limb Salvage Or Early Amputation
Limb Salvage or Early Amputation Evidence-Based Clinical Practice Guideline Adopted by: The American Academy of Orthopaedic Surgeons Board of Directors December 6, 2019 Endorsed by: Please cite this guideline as: American Academy of Orthopaedic Surgeons. Limb Salvage or Early Amputation Evidence-Based Clinical Practice Guideline. https://www.aaos.org/globalassets/quality-and-practice-resources/dod/ lsa-cpg-final-draft-12-10-19.pdf Published December 6, 2019 View background material via the LSA CPG eAppendix Disclaimer This clinical practice guideline was developed by a physician volunteer clinical practice guideline development group based on a formal systematic review of the available scientific and clinical information and accepted approaches to treatment and/or diagnosis. This clinical practice guideline is not intended to be a fixed protocol, as some patients may require more or less treatment or different means of diagnosis. Clinical patients may not necessarily be the same as those found in a clinical trial. Patient care and treatment should always be based on a clinician’s independent medical judgment, given the individual patient’s specific clinical circumstances. Disclosure Requirement In accordance with AAOS policy, all individuals whose names appear as authors or contributors to this clinical practice guideline filed a disclosure statement as part of the submission process. All panel members provided full disclosure of potential conflicts of interest prior to voting on the recommendations contained within this clinical practice guideline. Funding Source This clinical practice guideline was funded exclusively through a research grant provided by the United States Department of Defense with no funding from outside commercial sources to support the development of this document. -

Footprints an Informational Newsletter for Patients of APMA Member Podiatrists October 2017
footprints an informational newsletter for patients of APMA member podiatrists october 2017 special EDITION include a DPM for diabetes prevention & Manage Ment According to the CDC, more than 100 million US adults are living with either diabetes or prediabetes. If you have diabetes or prediabetes, it is essential to include a podiatrist for proper diabetes prevention and management. In fact, podiatrists can reduce amputation rates up to 80 percent. In order for you not to become a part of that statistic, here are a few simple things you can do to keep your risk for diabetic ulcers and amputation low: Inspect feet daily. Check your Don’t go barefoot. Don’t go 1 feet and toes every day for 5 without shoes, even in your cuts, bruises, sores, or changes to own home. The risk of cuts and the toenails, such as thickening or infection is too great for those discoloration. with diabetes. Wear thick, soft socks. Avoid Never try to remove calluses, 2 socks with seams, which could 6 corns, or warts by yourself. rub and cause blisters or other Over-the-counter products can burn skin injuries. the skin and cause irreparable damage to the foot for people Exercise. Walking can keep with diabetes. 3 weight down and improve circulation. Be sure to wear See a podiatrist. Regular appropriate athletic shoes when 7 checkups by a podiatrist— exercising. at least annually—are the best WALKING CAN KEEP way to ensure that your feet WEIGHT DOWN AND Have new shoes properly remain healthy. 4 measured and fitted. Foot size IMPROVE CIRCULATION. -
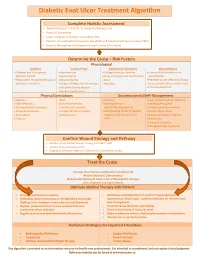
Diabetic Foot Ulcer Treatment Algorithm
Diabetic Foot Ulcer Treatment Algorithm Complete Holistic Assessment Medical/Surgical History & Co-morbidity Management Physical Examination Lower Leg (LLA) including monofilament test Perform Arterial Brachial Pressure Index (ABPI) and Toe Brachial Pressure Index (TBPI) Diabetic Management (Glycemic and Lipid Control & Nutrition) Determine the Cause – Risk Factors Physiological Diabetes Vascular Flow Autoimmune Disorders Wound History Diabetes and sub-optimal Hypertension Collagen vascular diseases History of foot infections or glycemic control Heart disease Immunosuppressant medications osteomyelitis Neuropathic changes with lack of Hyperlipidemia Gout Presence of toe infections (fungal protective sensation History of deep vein thrombosis Vasculitis or bacterial), callous and/or corns Peripheral artery disease Previous ulceration Venous Insufficiency Physical Limitations Socioeconomic/Self-Management Obesity Gait Smoking Lack of awareness for self-care Foot deformity Nutritional deficits Inadequate foot Inadequate hygiene Decreased level of activity Limited joint mobility wear/offloading devices Unsafe home environment Visual disturbances Congenital abnormalities Lack/Inability to afford diabetic Alcohol/drug abuse Amputation Osteoporosis supplies, foot care and foot Decreased Cognitive Ability Trauma wear Depression Financial insecurity Decreased level of activity Confirm Wound Etiology and Pathway Results of LLA, Monofilament Testing and ABPI/ TBPI Results of wound assessment Diagnostic -

Associations Between Nutrients and Foot Ulceration in Diabetes: a Systematic Review
nutrients Review Associations between Nutrients and Foot Ulceration in Diabetes: A Systematic Review Nada Bechara 1,2,3, Jenny E. Gunton 2,3,4,* , Victoria Flood 5,6 , Tien-Ming Hng 1 and Clare McGloin 1 1 Department of Diabetes and Endocrinology, Blacktown-Mt Druitt Hospital, Blacktown, NSW 2148, Australia; [email protected] (N.B.); [email protected] (T.-M.H.); [email protected] (C.M.) 2 Westmead Hospital, Sydney Medical School, Faculty of Medicine and Health, The University of Sydney, Westmead, NSW 2145, Australia 3 Centre for Diabetes, Obesity and Endocrinology Research (CDOER), The Westmead Institute for Medical Research, The University of Sydney, Westmead, NSW 2145, Australia 4 Garvan Institute of Medical Research, Darlinghurst, NSW 2010, Australia 5 Westmead Hospital, Research and Education Network, Western Sydney Local Health District, Westmead, NSW 2145, Australia; [email protected] 6 Faculty of Medicine and Health, Sydney School of Health Sciences, The University of Sydney, Camperdown, NSW 2006, Australia * Correspondence: [email protected] Abstract: We reviewed the literature to evaluate potential associations between vitamins, nutrients, nutritional status or nutritional interventions and presence or healing of foot ulceration in diabetes. Embase, Medline, PubMed, and the Cochrane Library were searched for studies published prior to September 2020. We assessed eligible studies for the association between nutritional status or interventions and foot ulcers. Fifteen studies met the inclusion criteria and were included in this Citation: Bechara, N.; Gunton, J.E.; review. Overall, there is a correlation between poor nutritional status and the presence of foot Flood, V.; Hng, T.-M.; McGloin, C. -

En Bloc Resection of Extra-Peritoneal Soft Tissue Neoplasms Incorporating a Type III Internal Hemipelvectomy: a Novel Approach Sanjay S Reddy1* and Norman D Bloom2
Reddy and Bloom World Journal of Surgical Oncology 2012, 10:222 http://www.wjso.com/content/10/1/222 WORLD JOURNAL OF SURGICAL ONCOLOGY REVIEW Open Access En bloc resection of extra-peritoneal soft tissue neoplasms incorporating a type III internal hemipelvectomy: a novel approach Sanjay S Reddy1* and Norman D Bloom2 Abstract Background: A type III hemipelvectomy has been utilized for the resection of tumors arising from the superior or inferior pubic rami. Methods: In eight patients, we incorporated a type III internal hemipelvectomy to achieve an en bloc R0 resection for tumors extending through the obturator foramen or into the ischiorectal fossa. The pelvic ring was reconstructed utilizing marlex mesh. This allowed for pelvic stability and abdominal wall reconstruction with obliteration of the obturator space to prevent herniations. Results: All eight patients had an R0 resection with an overall survival of 88% and with average follow up of 9.5 years. Functional evaluation utilizing the Enneking classification system, which evaluates motion, pain, stability and strength of the affected extremity, revealed a 62% excellent result and a 37% good result. No significant complications were associated with the operative procedure. Marlex mesh reconstruction provided pelvic stability and eliminated all hernial defects. Conclusion: The superior and inferior pubic rami provide a barrier to a resection for tumors that arise in the extra-peritoneal pelvis extending through the obturator foramen or ischiorectal fossa. Incorporating a type III internal -

Use of Anterolateral Thigh Flap for Reconstruction of Traumatic Bilateral Hemipelvectomy After Major Pelvic Trauma
Al‑wageeh et al. surg case rep (2020) 6:247 https://doi.org/10.1186/s40792‑020‑01009‑2 CASE REPORT Open Access Use of anterolateral thigh fap for reconstruction of traumatic bilateral hemipelvectomy after major pelvic trauma: a case report Saleh Al‑wageeh1 , Faisal Ahmed2* , Khalil Al‑naggar3 , Mohammad Reza Askarpour4 and Ebrahim Al‑shami5 Abstract Background: Major pelvic trauma (MPT) with traumatic hemipelvectomy (THP) is rare, but it is a catastrophic health problem caused by high‑energy injury leading to separation of the lower extremity from the axial skeleton, which is associated with a high incidence of intra‑abdominal and multi‑systemic injuries. THP is generally performed as a lifesaving protocol to return the patient to an active life. Case report: A 12‑year male patient exposed to major pelvic trauma with bilateral THP survived the trauma and mul‑ tiple lifesaving operations. The anterolateral thigh fap is the method used for wound reconstruction. The follow‑up was ended with colostomy and cystostomy with wheelchair mobilization. To the best of our knowledge, there have been a few bilateral THP reports, and our case is the second one to be successfully treated with an anterolateral thigh fap. Conclusion: MPT with THP is the primary cause of death among trauma patients. Life‑threatening hemorrhage is the usual cause of death, which is a strong indication for THP to save life. Keywords: Amputation, Hemipelvectomy, Myocutaneous fap, Reconstruction, Trauma Introduction A few victims survive these injuries, and the actual Major pelvic trauma (MPT) associated with traumatic incidence is unknown, but it is usually underestimated hemipelvectomy (THP) was described frst by Turnbull in [3]. -

Diabetic Ulcer Identification & Treatment
Diabetic Ulcer Identification & Treatment Bill Richlen PT, WCC, DWC, Clinical Instructor Wound Care Education Institute The information in this handout reflects the state of knowledge, current at time of publication; however, the authors do not take responsibility for data, information, and significant findings related to the topics discussed that became known to the general public following publication. The recommendations contained herein may not be appropriate for use in all circumstances. Decisions to adopt any particular recommendation must be made by the practitioner in light of available resources and circumstances presented by individual patients. Acknowledgements: This handout and presentation was prepared using information generally acknowledged to be consistent with current industry standards. The authors whose works are cited in the Bibliography Section of this manual are hereby recognized and appreciated. All product names, logos and trademarks used in this presentation are the property of the respective trademark owners. ® and ™ denote registered trademarks in the United States and other countries. "The use of NPUAP/EPUAP/PPPIA material does not imply endorsement of products or programs associated with the use of the material." Wound Care Education Institute (WCEI) Phone: 877-462-9234 Email: [email protected] www.wcei.net Copyright © 2018 by Wound Care Education Institute All Rights Reserved. WCEI grants permission for photocopying for limited personal or educational use only. This consent does not extend to other kinds of copying, such as copying for general distribution, for advertising or promotional purposes, for creating new collective works, or for resale. Treating Chronic Diabetic Wounds Outline I. Chronic Wounds A. Some Details 1. Definition: A wound that has failed to proceed orderly through the healing process or fails to result in durable closure.3 a. -

Clinician Assessment Tools for Patients with Diabetic Foot Disease: a Systematic Review
Journal of Clinical Medicine Review Clinician Assessment Tools for Patients with Diabetic Foot Disease: A Systematic Review Raúl Fernández-Torres 1 , María Ruiz-Muñoz 1,* , Alberto J. Pérez-Panero 1 , Jerónimo C. García-Romero 2 and Manuel Gónzalez-Sánchez 3 1 Department of Nursing and Podiatry, University of Málaga, Arquitecto Francisco Peñalosa, s/n. Ampliación Campus de Teatinos, 29071 Málaga, Spain; [email protected] (R.F.-T.); [email protected] (A.J.P.-P.) 2 Medical School of Physical Education and Sports, University of Málaga, C/Jiménez Fraud 10. Edificio López de Peñalver, 29010 Málaga, Spain; [email protected] 3 Department of Physiotherapy, University of Málaga, Arquitecto Francisco Peñalosa, s/n. Ampliación campus de Teatinos, 29071 Málaga, Spain; [email protected] * Correspondence: [email protected]; Tel.: +34-951953215 Received: 10 April 2020; Accepted: 12 May 2020; Published: 15 May 2020 Abstract: The amputation rate in patients with diabetes is 15 to 40 times higher than in patients without diabetes. To avoid major complications, the identification of high-risk in patients with diabetes through early assessment highlights as a crucial action. Clinician assessment tools are scales in which clinical examiners are specifically trained to make a correct judgment based on patient outcomes that helps to identify at-risk patients and monitor the intervention. The aim of this study is to carry out a systematic review of valid and reliable Clinician assessment tools for measuring diabetic foot disease-related variables and analysing their psychometric properties. The databases used were PubMed, Scopus, SciELO, CINAHL, Cochrane, PEDro, and EMBASE. The search terms used were foot, ankle, diabetes, diabetic foot, assessment, tools, instruments, score, scale, validity, and reliability. -
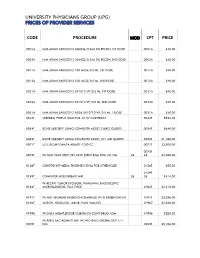
Code Procedure Cpt Price University Physicians Group
UNIVERSITY PHYSICIANS GROUP (UPG) PRICES OF PROVIDER SERVICES CODE PROCEDURE MOD CPT PRICE 0001A IMM ADMN SARSCOV2 30MCG/0.3ML DIL RECON 1ST DOSE 0001A $40.00 0002A IMM ADMN SARSCOV2 30MCG/0.3ML DIL RECON 2ND DOSE 0002A $40.00 0011A IMM ADMN SARSCOV2 100 MCG/0.5 ML 1ST DOSE 0011A $40.00 0012A IMM ADMN SARSCOV2 100 MCG/0.5 ML 2ND DOSE 0012A $40.00 0021A IMM ADMN SARSCOV2 5X1010 VP/0.5 ML 1ST DOSE 0021A $40.00 0022A IMM ADMN SARSCOV2 5X1010 VP/0.5 ML 2ND DOSE 0022A $40.00 0031A IMM ADMN SARSCOV2 AD26 5X10^10 VP/0.5 ML 1 DOSE 0031A $40.00 0042T CEREBRAL PERFUS ANALYSIS, CT W/CONTRAST 0042T $954.00 0054T BONE SURGERY USING COMPUTER ASSIST, FLURO GUIDED 0054T $640.00 0055T BONE SURGERY USING COMPUTER ASSIST, CT/ MRI GUIDED 0055T $1,188.00 0071T U/S LEIOMYOMATA ABLATE <200 CC 0071T $2,500.00 0075T 0075T PR TCAT PLMT XTRC VRT CRTD STENT RS&I PRQ 1ST VSL 26 26 $2,208.00 0126T CAROTID INT-MEDIA THICKNESS EVAL FOR ATHERSCLER 0126T $55.00 0159T 0159T COMPUTER AIDED BREAST MRI 26 26 $314.00 PR RECTAL TUMOR EXCISION, TRANSANAL ENDOSCOPIC 0184T MICROSURGICAL, FULL THICK 0184T $2,315.00 0191T PR ANT SEGMENT INSERTION DRAINAGE W/O RESERVOIR INT 0191T $2,396.00 01967 ANESTH, NEURAXIAL LABOR, PLAN VAG DEL 01967 $2,500.00 01996 PR DAILY MGMT,EPIDUR/SUBARACH CONT DRUG ADM 01996 $285.00 PR PERQ SAC AGMNTJ UNI W/WO BALO/MCHNL DEV 1/> 0200T NDL 0200T $5,106.00 PR PERQ SAC AGMNTJ BI W/WO BALO/MCHNL DEV 2/> 0201T NDLS 0201T $9,446.00 PR INJECT PLATELET RICH PLASMA W/IMG 0232T HARVEST/PREPARATOIN 0232T $1,509.00 0234T PR TRANSLUMINAL PERIPHERAL ATHERECTOMY, RENAL -

Dynamic Approaches to Improve Glycemic Control and Primary Diabetes Care 1‑Or 2‑Or 3‑Or
DYNAMIC APPROACHES TO IMPROVE GLYCEMIC CONTROL AND PRIMARY DIABETES CARE DYNAMIC APPROACHES TO IMPROVE GLYCEMIC Figure. CONTROL AND PRIMARY DIABETES CARE 1‑OR Characterizing Clinical Inertia in a Large, National Database CORI R. RATTELMAN, ANUPAMA ARORA, JOHN K. CUDDEBACK, ELIZABETH L. CIEMINS, Alexandria, VA Objective: To characterize clinical inertia in the treatment of diabetes using a large, geographically diverse clinical database. Study Design: A retrospective descriptive analysis was conducted in a clinical database containing 22 million patient records across 22 health care organizations (HCOs). Population Studied: A total of 281,000 patients aged 18-75 were included during the 5.5-year study period (1/2012-6/2017). Patients had an outpatient visit in the last 12 months of the study period, an HbA1c in the last 24-30 months (index A1c), and a diagnosis of type 2 DM on a claim or EHR prob- lem list at least 6 months prior to index A1c. A subset of 47,693 patients with an index A1c ≥8 and a prior A1c ≥8 or lack thereof, was observed for Supported By: National Institute of Diabetes and Digestive and Kidney Diseases four 6-month follow-up periods for actions including a new class of diabetes (T35DK104689); Yale University medication prescribed or an A1c <8. The absence of observable action fol- lowing index A1c suggests potential “clinical inertia.” Principal Findings: Six months following an index A1c≥8, 55% of patients 3‑OR received no observable clinical action ranging from 45-65% across HCOs Influence of Diabetes Complications on the Cost‑Effectiveness of and 18-96% across individual providers. -

Cross-Sectional Imaging in the Evaluation of Osteogenic Sarcoma: MRI and CT
Cross-sectional Imaging in the Evaluation of Osteogenic Sarcoma: MRI and CT By Leanne L. Seeger, Jeffrey J. Eckardt, and Lawrence W. Bassett EFORE the advent of cross-sectional scan- for treatment. Magnetic resonance imaging and B ning techniques, imaging of malignant bone CT image acquisition should address specific tumors was confined to plain radiography and issues that are important to the surgeon and radionuclide bone scanning. While radiography should be tailored according to tumor location remains the primary imaging modality for differ- and proposed surgical treatment, whether it be ential diagnosis, magnetic resonance imaging amputation or resection with limb salvage proce- (MRI) and computed tomography (CT) have dure. had a profound impact on the preoperative stag- The following discussion reflects the scanning ing evaluation of bone tumors and their response principles and techniques we use for evaluating to therapy. patients with OGS both preoperatively and post- In this article we will define the role of MRI operatively. This was derived from both our own and CT in the work-up of the patient with known experience and the experience of others as re- osteogenic sarcoma (OGS), stressing imaging ported in the literature. ‘-’ Regional differences in strategies that optimize information available to the management of patients with malignant bone the clinician and assist in therapy planning. In tumors may alter this procedure. To become order to achieve these optimal factors, communi- familiar with local treatment protocols, commu- cation with the referring physician and review of nicate directly with the surgeon who will ulti- available radiographs and radionuclide bone scans mately be responsible for treatment. -

ICD-9-CM Procedures (FY10)
2 PREFACE This sixth edition of the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) is being published by the United States Government in recognition of its responsibility to promulgate this classification throughout the United States for morbidity coding. The International Classification of Diseases, 9th Revision, published by the World Health Organization (WHO) is the foundation of the ICD-9-CM and continues to be the classification employed in cause-of-death coding in the United States. The ICD-9-CM is completely comparable with the ICD-9. The WHO Collaborating Center for Classification of Diseases in North America serves as liaison between the international obligations for comparable classifications and the national health data needs of the United States. The ICD-9-CM is recommended for use in all clinical settings but is required for reporting diagnoses and diseases to all U.S. Public Health Service and the Centers for Medicare & Medicaid Services (formerly the Health Care Financing Administration) programs. Guidance in the use of this classification can be found in the section "Guidance in the Use of ICD-9-CM." ICD-9-CM extensions, interpretations, modifications, addenda, or errata other than those approved by the U.S. Public Health Service and the Centers for Medicare & Medicaid Services are not to be considered official and should not be utilized. Continuous maintenance of the ICD-9- CM is the responsibility of the Federal Government. However, because the ICD-9-CM represents the best in contemporary thinking of clinicians, nosologists, epidemiologists, and statisticians from both public and private sectors, no future modifications will be considered without extensive advice from the appropriate representatives of all major users.