Dynamic Approaches to Improve Glycemic Control and Primary Diabetes Care 1‑Or 2‑Or 3‑Or
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evidenz Und Versorgungsrealität Von Kurzwirksamen Insulinanaloga in Der Behandlung Des Typ-2-Diabetes Mellitus
Evidenz und Versorgungsrealität von kurzwirksamen Insulinanaloga in der Behandlung des Typ-2-Diabetes mellitus – Eine Versorgungsanalyse auf der Basis von Sekundärdaten – Dissertation zur Erlangung des Doktorgrades der Naturwissenschaften vorgelegt beim Fachbereich 14 - Biochemie, Chemie und Pharmazie der Johann Wolfgang Goethe-Universität in Frankfurt am Main von Matthias S. Pfannkuche aus Brühl Frankfurt am Main, im Jahr 2009 vom Fachbereich 14 - Biochemie, Chemie und Pharmazie der Johann Wolfgang Goethe-Universität als Dissertation angenommen. Dekan: Prof. Dr. rer. nat. Dieter Steinhilber Gutachter: Prof. Dr. rer. nat. Theo Dingermann Prof. Dr. rer. nat. Gerd Glaeske (Universität Bremen) Datum der Disputation: 15. Februar 2010 Bildnachweis Titelseite: Insulin Hexamer: http://commons.wikimedia.org/wiki/File:Human-insulin-hexamer-3D-ribbons.png (letzter Zugriff: 11.06.2009) Non semper ea sunt, quae videntur. (Phädrus, fabulae 4, 2, 5) Danksagung Diese Dissertation sowie die hiermit in Verbindung stehenden Publikationen wären ohne die Anregungen und Unterstützung durch viele Kollegen, Freunde und Organisationen nicht möglich gewesen. Ihnen möchte ich an dieser Stelle danken. Mein besonderer Dank gilt Prof. Dr. rer. nat Gerd Glaeske und Prof. Dr. rer. nat. Theo Dingermann, die diese Arbeit in vielerlei Hinsicht erst ermöglichten. Überaus dankbar bin ich Prof. Dr. rer. nat. Gerd Glaeske für die freundliche Aufnahme in seine Arbeitsgruppe, die es mir ermöglichte weitere Einblicke in die Gesundheitsökonomie, Gesundheitspolitik und Versorgungsforschung zu nehmen. Für das Korrekturlesen der kompletten Arbeit, die zahlreichen Hinweise und konstruktiven Diskussionen sowie die zahlreichen Mittagspausen danke ich im besonderen Dr. P.H. Falk Hoffmann. Herzlicher Dank gilt auch dem gesamten Arbeitskreis in Bremen sowie den Projektbeteiligten Krankenkassen, allen voran der GEK, die mir durch den Zugriff auf ihre Daten erst viele Analysen ermöglichten. -
![LANTUS® (Insulin Glargine [Rdna Origin] Injection)](https://docslib.b-cdn.net/cover/0369/lantus%C2%AE-insulin-glargine-rdna-origin-injection-60369.webp)
LANTUS® (Insulin Glargine [Rdna Origin] Injection)
Rev. March 2007 Rx Only LANTUS® (insulin glargine [rDNA origin] injection) LANTUS® must NOT be diluted or mixed with any other insulin or solution. DESCRIPTION LANTUS® (insulin glargine [rDNA origin] injection) is a sterile solution of insulin glargine for use as an injection. Insulin glargine is a recombinant human insulin analog that is a long-acting (up to 24-hour duration of action), parenteral blood-glucose-lowering agent. (See CLINICAL PHARMACOLOGY). LANTUS is produced by recombinant DNA technology utilizing a non- pathogenic laboratory strain of Escherichia coli (K12) as the production organism. Insulin glargine differs from human insulin in that the amino acid asparagine at position A21 is replaced by glycine and two arginines are added to the C-terminus of the B-chain. Chemically, it is 21A- B B Gly-30 a-L-Arg-30 b-L-Arg-human insulin and has the empirical formula C267H404N72O78S6 and a molecular weight of 6063. It has the following structural formula: LANTUS consists of insulin glargine dissolved in a clear aqueous fluid. Each milliliter of LANTUS (insulin glargine injection) contains 100 IU (3.6378 mg) insulin glargine. Inactive ingredients for the 10 mL vial are 30 mcg zinc, 2.7 mg m-cresol, 20 mg glycerol 85%, 20 mcg polysorbate 20, and water for injection. Inactive ingredients for the 3 mL cartridge are 30 mcg zinc, 2.7 mg m-cresol, 20 mg glycerol 85%, and water for injection. The pH is adjusted by addition of aqueous solutions of hydrochloric acid and sodium hydroxide. LANTUS has a pH of approximately 4. CLINICAL PHARMACOLOGY Mechanism of Action: The primary activity of insulin, including insulin glargine, is regulation of glucose metabolism. -

Metabolism Lectures Diabetes
Metabolism Lectures Diabetes Dalay Olson Ph.D. Integrative Biology and Physiology Email: [email protected] Office: 3-120 Jackson Hall Learning Objectives 1. Compare and contrast type 1 and type 2 diabetes. Identify the major similarities and differences (insulin level, insulin signaling events, treatment options, glucose tolerance test responses etc.) 2. How is diabetes diagnosed in the clinic? What tests are available and commonly used? What information does a glucose tolerance give you? What information does a hemoglobin A1C tell you? What are the limitations of these tests? 3. Explain how chronic elevation of blood glucose levels can result in AGE formation and AGE receptor (RAGE) activation. Explain the downstream effects of RAGE activation. 4. Identify the role of PKC (Protein Kinase C) in arteriolar vasoconstriction in type 2 diabetics. 5. List the common symptoms you might see in the clinic for a patient suffering from type 2 diabetes. 6. Explain why the plasma becomes acidic in a patient with uncontrolled diabetes. 7. Explain the mechanism of action (what is the point of the drug?) of insulin sensitizers, insulin mimetics, insulin secretagogues and SGLT2 inhibitors. Natural Progression of Type-2-Diabetes Pre-diabetes Lean Obese Henry, Am J Med 1998; 105 (1A):20S-6S Think about what is happening with Type 1 diabetics…how does their response differ from that of a normal person? Glucose Insulin is not present, Insulin binds to receptor therefore the signal transduction pathway is not initiated and glucose Glucose transporters remain in enters cell Exocytosis intracellular vesicles. Signal transduction cascade Plasma glucose remains high! GLUT4 Think about what is happening with Type-2-diabetics…how does their response differ from that of a Type-1- diabetic? Glucose Insulin is present but is not Insulin binds to receptor functional, therefore the signal transduction pathway is not initiated and glucose Glucose transporters remain in Exocytosis enters cell intracellular vesicles. -

Combining a Glucagon-Like Peptide-1 Receptor Agonist with Basal Insulin: the Why and How
Combining a Glucagon-like Peptide-1 Receptor Agonist with Basal Insulin: The Why and How Case Study Mary is a 61 year-old female diagnosed with type 2 diabetes mellitus (T2DM) 8 years ago. She was initially managed with the combination of lifestyle modification and metformin. Since that time she was treated with a sulfonylurea, but it was discontinued due to symptomatic hypoglycemia. She was also treated with pioglitazone, but significant fluid retention led to it discontinuation. A year-and-a- half ago, basal insulin was added to her lifestyle and metformin management. She now administers 52 units (0.62 units/kg) once daily at bedtime. Since starting basal insulin, she has experienced 3 episodes of mild hypoglycemia. Since her diagnosis, Mary’s HbA1c has never been <7.0%; her current HbA1c is 7.9%. Over the past month, her fasting plasma glucose (FPG) has ranged from 103 mg/dL to 136 mg/dL and her postprandial glucose (PPG) from 164 mg/dL to 213 mg/dL. She has gained 2.6 kg since starting basal insulin and her body mass index is now 31 kg/m2. Her blood pressure is 134/82 mmHg. She experiences occasional tingling in her feet. Eye examination reveals grade 1 retinopathy. Current medications are: metformin 1000mg twice daily, basal insulin 52 units once daily at bedtime, and hydrochlorothiazide 25 mg once daily. Her family physician notes that Mary’s FPG is reasonably well-controlled, yet her HbA1c and PPG remain elevated. He is also concerned about her episodes of hypoglycemia and weight gain and the evidence indicating microvascular damage. -

Footprints an Informational Newsletter for Patients of APMA Member Podiatrists October 2017
footprints an informational newsletter for patients of APMA member podiatrists october 2017 special EDITION include a DPM for diabetes prevention & Manage Ment According to the CDC, more than 100 million US adults are living with either diabetes or prediabetes. If you have diabetes or prediabetes, it is essential to include a podiatrist for proper diabetes prevention and management. In fact, podiatrists can reduce amputation rates up to 80 percent. In order for you not to become a part of that statistic, here are a few simple things you can do to keep your risk for diabetic ulcers and amputation low: Inspect feet daily. Check your Don’t go barefoot. Don’t go 1 feet and toes every day for 5 without shoes, even in your cuts, bruises, sores, or changes to own home. The risk of cuts and the toenails, such as thickening or infection is too great for those discoloration. with diabetes. Wear thick, soft socks. Avoid Never try to remove calluses, 2 socks with seams, which could 6 corns, or warts by yourself. rub and cause blisters or other Over-the-counter products can burn skin injuries. the skin and cause irreparable damage to the foot for people Exercise. Walking can keep with diabetes. 3 weight down and improve circulation. Be sure to wear See a podiatrist. Regular appropriate athletic shoes when 7 checkups by a podiatrist— exercising. at least annually—are the best WALKING CAN KEEP way to ensure that your feet WEIGHT DOWN AND Have new shoes properly remain healthy. 4 measured and fitted. Foot size IMPROVE CIRCULATION. -

Performance of the Insulin-Only Ilet Bionic Pancreas and The
e118 Diabetes Care Volume 44, June 2021 Performance of the Insulin-Only Luz E. Castellanos,1 Courtney A. Balliro,1 Jordan S. Sherwood,1 Rabab Jafri,1 iLet Bionic Pancreas and the Mallory A. Hillard,1 Evelyn Greaux,1 Rajendranath Selagamsetty,2 Hui Zheng,3 Bihormonal iLet Using Firas H. El-Khatib,2 Edward R. Damiano,2,4 and Dasiglucagon in Adults With Type Steven J. Russell1 1 Diabetes in a Home-Use Setting Diabetes Care 2021;44:e118–e120 | https://doi.org/10.2337/dc20-1086 Reductions in blood glucose levels in with insulin lispro (Eli Lilly) or aspart (Table 1). The mean CGM glucose and people with diabetes are often achieved (Novo Nordisk), the bihormonal iLet for time in range (70–180 mg/dL) were 149 at the expense of increased hypoglyce- 7dayswithdasiglucagon(4mg/mL) ±13mg/dLand72±8%,respectively,in mia. A novel approach is to automati- and insulin lispro or aspart, or both, us- the insulin-only period, and 139 ± 11 cally deliver microdose glucagon when ing the same glucose target (110 mg/ mg/dL and 79 ± 9%, respectively, in the automation of insulin delivery alone is dL), in random order. There were no re- bihormonal period. The mean daily car- not sufficient to prevent hypoglycemia. strictions on diet or exercise. The prima- bohydrates consumed to prevent or The approach requires a bihormonal de- ry outcomes were prespecified iLet treat hypoglycemia were 16 ± 13 g and vice and a stable form of glucagon or operational thresholds. The key second- 18 ± 21 g in the insulin-only and bihor- glucagon analog. -

TRULICITY, INN-Dulaglutide
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Trulicity 0.75 mg solution for injection in pre-filled pen Trulicity 1.5 mg solution for injection in pre-filled pen Trulicity 3 mg solution for injection in pre-filled pen Trulicity 4.5 mg solution for injection in pre-filled pen 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Trulicity 0.75 mg solution for injection in pre-filled pen Each pre-filled pen contains 0.75 mg of dulaglutide* in 0.5 ml solution. Trulicity 1.5 mg solution for injection in pre-filled pen Each pre-filled pen contains 1.5 mg of dulaglutide* in 0.5 ml solution. Trulicity 3 mg solution for injection in pre-filled pen Each pre-filled pen contains 3 mg of dulaglutide* in 0.5 ml solution. Trulicity 4.5 mg solution for injection in pre-filled pen Each pre-filled pen contains 4.5 mg of dulaglutide* in 0.5 ml solution. *produced in CHO cells by recombinant DNA technology. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Solution for injection. Clear, colourless solution. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Type 2 Diabetes Mellitus Trulicity is indicated for the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise • as monotherapy when metformin is considered inappropriate due to intolerance or contraindications • in addition to other medicinal products for the treatment of diabetes. For study results with respect to combinations, effects on glycaemic control and cardiovascular events, and the populations studied, see sections 4.4, 4.5 and 5.1. -

Decrease of Fructosamine Levels During Treatment with Adalimumab
European Journal of Endocrinology (2007) 156 291–293 ISSN 0804-4643 CASE REPORT Decrease of fructosamine levels during treatment with adalimumab in patients with both diabetes and rheumatoid arthritis I C van Eijk1, M J L Peters1,2, M T Nurmohamed1,2,3, A W van Deutekom4, B A C Dijkmans1,2 and S Simsek4 1Department of Rheumatology, Jan van Breemen Institute, Amsterdam, The Netherlands, 2Department of Rheumatology, VU University Medical Center, Amsterdam, The Netherlands, 3Department of Internal Medicine, VU University Medical Center, Amsterdam, The Netherlands and 4Department of Endocrinology/Diabetes Center, VU University Medical Center, PO Box 7057, 1007 MB, Boelelaan 1117, Amsterdam, The Netherlands (Correspondence should be addressed to S Simsek; Email: [email protected]) Abstract Tumour necrosis factor a (TNFa) is a pro-inflammatory cytokine which has been closely linked to obesity and insulin resistance. We present two cases of patients with rheumatoid arthritis (RA) and concomitant diabetes mellitus, who showed a marked decrease of fructosamine levels after initiating therapy with adalimumab, a TNFa-blocking agent, for active RA. This finding may implicate that TNFa blockade causes better glycaemic control in RA patients with concomitant diabetes, possibly by improving insulin resistance. European Journal of Endocrinology 156 291–293 Introduction Research design and methods Tumour necrosis factor a (TNFa), a pro-inflammatory A patient with known diabetes type 1 and concomitant cytokine, plays an important role in inflammatory and RA showed a marked improvement of HbA1c levels after autoimmune diseases like rheumatoid arthritis (RA). initiation of adalimumab, a recombinant human IgG1- TNFa has also been closely linked to obesity and insulin MAB, therapy for active RA when she visited the resistance (1). -
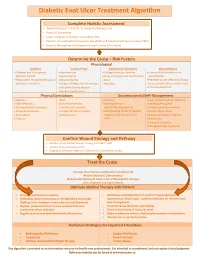
Diabetic Foot Ulcer Treatment Algorithm
Diabetic Foot Ulcer Treatment Algorithm Complete Holistic Assessment Medical/Surgical History & Co-morbidity Management Physical Examination Lower Leg (LLA) including monofilament test Perform Arterial Brachial Pressure Index (ABPI) and Toe Brachial Pressure Index (TBPI) Diabetic Management (Glycemic and Lipid Control & Nutrition) Determine the Cause – Risk Factors Physiological Diabetes Vascular Flow Autoimmune Disorders Wound History Diabetes and sub-optimal Hypertension Collagen vascular diseases History of foot infections or glycemic control Heart disease Immunosuppressant medications osteomyelitis Neuropathic changes with lack of Hyperlipidemia Gout Presence of toe infections (fungal protective sensation History of deep vein thrombosis Vasculitis or bacterial), callous and/or corns Peripheral artery disease Previous ulceration Venous Insufficiency Physical Limitations Socioeconomic/Self-Management Obesity Gait Smoking Lack of awareness for self-care Foot deformity Nutritional deficits Inadequate foot Inadequate hygiene Decreased level of activity Limited joint mobility wear/offloading devices Unsafe home environment Visual disturbances Congenital abnormalities Lack/Inability to afford diabetic Alcohol/drug abuse Amputation Osteoporosis supplies, foot care and foot Decreased Cognitive Ability Trauma wear Depression Financial insecurity Decreased level of activity Confirm Wound Etiology and Pathway Results of LLA, Monofilament Testing and ABPI/ TBPI Results of wound assessment Diagnostic -

Mechanism of Satellite Cell Regulation by Myokines
J Phys Fitness Sports Med, 6 (5): 311-316 (2017) DOI: 10.7600/jpfsm.6.311 JPFSM: Review Article Mechanism of satellite cell regulation by myokines Yasuro Furuichi* and Nobuharu L. Fujii Department of Health Promotion Sciences, Graduate School of Human Health Sciences, Tokyo Metropolitan University, 1-1 Minami-Osawa, Hachioji, Tokyo 193-0397, Japan Received: July 19, 2017 / Accepted: August 7, 2017 Abstract Skeletal muscle stem cells, known as satellite cells, participate in postnatal skeletal muscle growth, regeneration, and hypertrophy. They are quiescent in the resting state, but are activated after muscle injury, and subsequently replicate and fuse into existing myofibers. The behavior of satellite cells during muscle regeneration is regulated by extrinsic factors, such as the extracellular matrix, mechanical stimuli, and soluble factors. Myokines, muscle-derived secretory factors, are important regulators of satellite cell activation, proliferation, and differen- tiation. It is well known that muscle injury induces the release of various growth factors from myofibers, and these growth factors affect satellite cells. It has recently been shown that myo- kines secreted from myofibers without cell damage also regulate satellite cell functions. Here, we summarize myokines with known roles in the regulation of satellite cells and the mecha- nism underlying this regulatory process. Keywords : secretion, myogenesis, muscle regeneration regulation, but there are also molecules related to muscle Introduction regeneration or plasticity. In this review, we introduce the Skeletal muscle is a unique tissue that has a remarkable reported myokines involved in the regulation of satellite ability to regenerate after injury. In response to tissue cell behavior and its molecular mechanism. -

Management of Hyperglycaemia and Steroid (Glucocorticoid) Therapy
Management of Hyperglycaemia and Steroid (Glucocorticoid) Therapy October 2014 This document is coded JBDS 08 in the series of JBDS documents Other JBDS documents: Admissions avoidance and diabetes: guidance for clinical commissioning groups and clinical team; December 2013, JBDS 07 The management of the hyperosmolar hyperglycaemic state (HHS) in adults with diabetes; August 2012, JBDS 06 Glycaemic management during the inpatient enteral feeding of stroke patients with diabetes; June 2012, JBDS 05 Self-management of diabetes in hospital; March 2012, JBDS 04 Management of adults with diabetes undergoing surgery and elective procedures: improving standards; April 2011, JBDS 03 The Management of Diabetic Ketoacidosis in Adults; revised September 2013, JBDS 02 The hospital management of hypoglycaemia in adults with diabetes mellitus; revised September 2013, JBDS 01 These documents are available to download from: ABCD website: www.diabetologists-abcd.org.uk/JBDS/JBDS.htm Diabetes UK website: www.diabetes.org.uk Contents Page Foreword 4 Authorship and acknowledgments 5-6 Introduction 7 Steroids - mechanism of action 8 Steroid therapy – impact on blood glucose 9 Glucose targets 10 Glucose monitoring 11 Diabetes treatment options 12-13 Treatment of steroid induced hyperglycaemia 14-15 Hospital discharge 16-17 Steroid treatment in pregnancy 18 Steroid treatment in end of life 19 Audit standards 20 Controversial areas 21 References 22 Appendix 1 – Algorithm to show treatment of steroid 23 induced diabetes Appendix 2 – Algorithm to show management of patients 24 with diabetes on once daily steroids Appendix 3 – End of life steroid management 25 Appendix 4 – Patient letter – Glucose monitoring and 26 steroid use 3 Foreword This is the latest in the series of Joint British Diabetes Societies for Inpatient Care (JBDS-IP) guidelines, and focuses on steroid induced hyperglycaemia and steroid induced diabetes. -

Physical Exercise and Myokines: Relationships with Sarcopenia and Cardiovascular Complications
International Journal of Molecular Sciences Review Physical Exercise and Myokines: Relationships with Sarcopenia and Cardiovascular Complications Sandra Maria Barbalho 1,2,3,* , Uri Adrian Prync Flato 1,2 , Ricardo José Tofano 1,2, Ricardo de Alvares Goulart 1, Elen Landgraf Guiguer 1,2,3 , Cláudia Rucco P. Detregiachi 1 , Daniela Vieira Buchaim 1,4, Adriano Cressoni Araújo 1,2 , Rogério Leone Buchaim 1,5, Fábio Tadeu Rodrigues Reina 1, Piero Biteli 1, Daniela O. B. Rodrigues Reina 1 and Marcelo Dib Bechara 2 1 Postgraduate Program in Structural and Functional Interactions in Rehabilitation, University of Marilia (UNIMAR), Avenue Hygino Muzzy Filho, 1001, Marília 17525-902, São Paulo, Brazil; urifl[email protected] (U.A.P.F.); [email protected] (R.J.T.); [email protected] (R.d.A.G.); [email protected] (E.L.G.); [email protected] (C.R.P.D.); [email protected] (D.V.B.); [email protected] (A.C.A.); [email protected] (R.L.B.); [email protected] (F.T.R.R.); [email protected] (P.B.); [email protected] (D.O.B.R.R.) 2 School of Medicine, University of Marília (UNIMAR), Avenida Higino Muzzi Filho, 1001, Marília 17506-000, São Paulo, Brazil; [email protected] 3 Department of Biochemistry and Nutrition, Food Technology School, Marília 17525-902, São Paulo, Brazil 4 Medical School, University Center of Adamantina (UniFAI), Adamantina 17800-000, São Paulo, Brazil 5 Department of Biological Sciences, Bauru School of Dentistry, University of São Paulo (FOB–USP), Alameda Doutor Octávio Pinheiro Brisolla, 9-75, Bauru 17012901, São Paulo, Brazil * Correspondence: [email protected]; Tel.: +55-14-99655-3190 Received: 6 May 2020; Accepted: 19 May 2020; Published: 20 May 2020 Abstract: Skeletal muscle is capable of secreting different factors in order to communicate with other tissues.