Provider Type 72 Nurse Anesthetist Reimbursement Rates
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clinical Practice Guideline for Limb Salvage Or Early Amputation
Limb Salvage or Early Amputation Evidence-Based Clinical Practice Guideline Adopted by: The American Academy of Orthopaedic Surgeons Board of Directors December 6, 2019 Endorsed by: Please cite this guideline as: American Academy of Orthopaedic Surgeons. Limb Salvage or Early Amputation Evidence-Based Clinical Practice Guideline. https://www.aaos.org/globalassets/quality-and-practice-resources/dod/ lsa-cpg-final-draft-12-10-19.pdf Published December 6, 2019 View background material via the LSA CPG eAppendix Disclaimer This clinical practice guideline was developed by a physician volunteer clinical practice guideline development group based on a formal systematic review of the available scientific and clinical information and accepted approaches to treatment and/or diagnosis. This clinical practice guideline is not intended to be a fixed protocol, as some patients may require more or less treatment or different means of diagnosis. Clinical patients may not necessarily be the same as those found in a clinical trial. Patient care and treatment should always be based on a clinician’s independent medical judgment, given the individual patient’s specific clinical circumstances. Disclosure Requirement In accordance with AAOS policy, all individuals whose names appear as authors or contributors to this clinical practice guideline filed a disclosure statement as part of the submission process. All panel members provided full disclosure of potential conflicts of interest prior to voting on the recommendations contained within this clinical practice guideline. Funding Source This clinical practice guideline was funded exclusively through a research grant provided by the United States Department of Defense with no funding from outside commercial sources to support the development of this document. -

CPT® New Codes 2019: Biopsy, Skin
Billing and Coding Update Alexander Miller, M.D. AAD Representative to the AMA CPT Advisory Committee New Skin Biopsy CPT® Codes It’s all about the Technique! SPEAKER: Alexander Miller, M.D. AAD Representative to the AMA -CPT Advisory Committee Chair AAD Health Care Finance Committee Arriving on January 1, 2019 New and Restructured Biopsy Codes Tangential biopsy Punch Biopsy Incisional Biopsy How Did We Get Here? CMS CY 2016 Biopsy codes (11100, 11101 identified as potentially mis-valued; high expenditure RUC Survey sent to AAD Members Specialty survey results are the only tool available to support code values Challenging survey results Survey revealed bimodal data distribution; CPT Codes 11100, 11101 referred to CPT for respondents were valuing different procedures restructuring Rationale for New Codes 11100; 11101 • Previous skin biopsy codes did not distinguish between the different biopsy techniques that were being used CPT Recommended technique specification in new biopsy codes • Will also provide for reimbursement commensurate with the technique used How Did We Get Here? • CPT Editorial Panel deleted 11100; 11101 February 2017 • 6 New codes created based on technique utilized • Each technique: primary code and add-on code March 2017 • RUC survey sent to AAD members April 2017 • Survey results presented to the RUC Biopsy Codes Effective Jan., 1, 2019 • Integumentary biopsy codes 11755 Biopsy of nail unit (plate, bed, matrix, hyponychium, proximal and lateral nail folds 11100, 11101 have been deleted 30100 Biopsy, intranasal • New -

ANMC Specialty Clinic Services
Cardiology Dermatology Diabetes Endocrinology Ear, Nose and Throat (ENT) Gastroenterology General Medicine General Surgery HIV/Early Intervention Services Infectious Disease Liver Clinic Neurology Neurosurgery/Comprehensive Pain Management Oncology Ophthalmology Orthopedics Orthopedics – Back and Spine Podiatry Pulmonology Rheumatology Urology Cardiology • Cardiology • Adult transthoracic echocardiography • Ambulatory electrocardiology monitor interpretation • Cardioversion, electrical, elective • Central line placement and venous angiography • ECG interpretation, including signal average ECG • Infusion and management of Gp IIb/IIIa agents and thrombolytic agents and antithrombotic agents • Insertion and management of central venous catheters, pulmonary artery catheters, and arterial lines • Insertion and management of automatic implantable cardiac defibrillators • Insertion of permanent pacemaker, including single/dual chamber and biventricular • Interpretation of results of noninvasive testing relevant to arrhythmia diagnoses and treatment • Hemodynamic monitoring with balloon flotation devices • Non-invasive hemodynamic monitoring • Perform history and physical exam • Pericardiocentesis • Placement of temporary transvenous pacemaker • Pacemaker programming/reprogramming and interrogation • Stress echocardiography (exercise and pharmacologic stress) • Tilt table testing • Transcutaneous external pacemaker placement • Transthoracic 2D echocardiography, Doppler, and color flow Dermatology • Chemical face peels • Cryosurgery • Diagnosis -

En Bloc Resection of Extra-Peritoneal Soft Tissue Neoplasms Incorporating a Type III Internal Hemipelvectomy: a Novel Approach Sanjay S Reddy1* and Norman D Bloom2
Reddy and Bloom World Journal of Surgical Oncology 2012, 10:222 http://www.wjso.com/content/10/1/222 WORLD JOURNAL OF SURGICAL ONCOLOGY REVIEW Open Access En bloc resection of extra-peritoneal soft tissue neoplasms incorporating a type III internal hemipelvectomy: a novel approach Sanjay S Reddy1* and Norman D Bloom2 Abstract Background: A type III hemipelvectomy has been utilized for the resection of tumors arising from the superior or inferior pubic rami. Methods: In eight patients, we incorporated a type III internal hemipelvectomy to achieve an en bloc R0 resection for tumors extending through the obturator foramen or into the ischiorectal fossa. The pelvic ring was reconstructed utilizing marlex mesh. This allowed for pelvic stability and abdominal wall reconstruction with obliteration of the obturator space to prevent herniations. Results: All eight patients had an R0 resection with an overall survival of 88% and with average follow up of 9.5 years. Functional evaluation utilizing the Enneking classification system, which evaluates motion, pain, stability and strength of the affected extremity, revealed a 62% excellent result and a 37% good result. No significant complications were associated with the operative procedure. Marlex mesh reconstruction provided pelvic stability and eliminated all hernial defects. Conclusion: The superior and inferior pubic rami provide a barrier to a resection for tumors that arise in the extra-peritoneal pelvis extending through the obturator foramen or ischiorectal fossa. Incorporating a type III internal -

A Clinical and Histological Study of Radiofrequency-Assisted Liposuction (RFAL) Mediated Skin Tightening and Cellulite Improvement ——RFAL for Skin Tightening
Journal of Cosmetics, Dermatological Sciences and Applications, 2011, 1, 36-42 doi:10.4236/jcdsa.2011.12006 Published Online June 2011 (http://www.SciRP.org/journal/jcdsa) A Clinical and Histological Study of Radiofrequency-Assisted Liposuction (RFAL) Mediated Skin Tightening and Cellulite Improvement ——RFAL for Skin Tightening Marc Divaris1, Sylvie Boisnic2, Marie-Christine Branchet2, Malcolm D. Paul3 1Plastic and Maxillo-Facial Surgery, University of Pitie Salpetiere, Paris, France; 2Institution GREDECO, Paris, France; 3Department of Surgery, Aesthetic and PlasticSurgery Institute, University of California, Irvine, USA. Email: [email protected] Received May 1st, 2011; revised May 27th, 2011; accepted June 6th, 2011. ABSTRACT Background: A novel Radiofrequency-Assisted Liposuction (RFAL) technology was evaluated clinically. Parallel origi- nal histological studies were conducted to substantiate the technology’s efficacy in skin tightening, and cellulite im- provement. Methods: BodyTiteTM system, utilizing the RFAL technology, was used for treating patients on abdomen, hips, flanks and arms. Clinical results were measured on 53 patients up to 6 months follow-up. Histological and bio- chemical studies were conducted on 10 donors by using a unique GREDECO model of skin fragments cultured under survival conditions. Fragments from RFAL treated and control areas were examined immediately and after 10 days in culture, representing long-term results. Skin fragments from patients with cellulite were also examined. Results: Grad- ual improvement in circumference reduction (3.9 - 4.9 cm) and linear contraction (8% - 38%) was observed until the third month. These results stabilized at 6 months. No adverse events were recorded. Results were graded as excellent by most patients, including the satisfaction from minimal pain, bleeding, and downtime. -

Co™™I™™Ee Opinion
The American College of Obstetricians and Gynecologists WOMEN’S HEALTH CARE PHYSICIANS COMMITTEE OPINION Number 673 • September 2016 (Replaces Committee Opinion No. 345, October 2006) Committee on Gynecologic Practice This Committee Opinion was developed by the American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice and the American Society for Colposcopy and Cervical Pathology (ASCCP) in collaboration with committee member Ngozi Wexler, MD, MPH, and ASCCP members and experts Hope K. Haefner, MD, Herschel W. Lawson, MD, and Colleen K. Stockdale, MD, MS. This document reflects emerging clinical and scientific advances as of the date issued and is subject to change. The information should not be construed as dictating an exclusive course of treatment or procedure to be followed. Persistent Vulvar Pain ABSTRACT: Persistent vulvar pain is a complex disorder that frequently is frustrating to the patient and the clinician. It can be difficult to treat and rapid resolution is unusual, even with appropriate therapy. Vulvar pain can be caused by a specific disorder or it can be idiopathic. Idiopathic vulvar pain is classified as vulvodynia. Although optimal treatment remains unclear, consider an individualized, multidisciplinary approach to address all physical and emotional aspects possibly attributable to vulvodynia. Specialists who may need to be involved include sexual counselors, clinical psychologists, physical therapists, and pain specialists. Patients may perceive this approach to mean the practitioner does not believe their pain is “real”; thus, it is important to begin any treatment approach with a detailed discussion, including an explanation of the diagnosis and determination of realistic treatment goals. Future research should aim at evaluating a multimodal approach in the treatment of vulvodynia, along with more research on the etiologies of vulvodynia. -

Use of Anterolateral Thigh Flap for Reconstruction of Traumatic Bilateral Hemipelvectomy After Major Pelvic Trauma
Al‑wageeh et al. surg case rep (2020) 6:247 https://doi.org/10.1186/s40792‑020‑01009‑2 CASE REPORT Open Access Use of anterolateral thigh fap for reconstruction of traumatic bilateral hemipelvectomy after major pelvic trauma: a case report Saleh Al‑wageeh1 , Faisal Ahmed2* , Khalil Al‑naggar3 , Mohammad Reza Askarpour4 and Ebrahim Al‑shami5 Abstract Background: Major pelvic trauma (MPT) with traumatic hemipelvectomy (THP) is rare, but it is a catastrophic health problem caused by high‑energy injury leading to separation of the lower extremity from the axial skeleton, which is associated with a high incidence of intra‑abdominal and multi‑systemic injuries. THP is generally performed as a lifesaving protocol to return the patient to an active life. Case report: A 12‑year male patient exposed to major pelvic trauma with bilateral THP survived the trauma and mul‑ tiple lifesaving operations. The anterolateral thigh fap is the method used for wound reconstruction. The follow‑up was ended with colostomy and cystostomy with wheelchair mobilization. To the best of our knowledge, there have been a few bilateral THP reports, and our case is the second one to be successfully treated with an anterolateral thigh fap. Conclusion: MPT with THP is the primary cause of death among trauma patients. Life‑threatening hemorrhage is the usual cause of death, which is a strong indication for THP to save life. Keywords: Amputation, Hemipelvectomy, Myocutaneous fap, Reconstruction, Trauma Introduction A few victims survive these injuries, and the actual Major pelvic trauma (MPT) associated with traumatic incidence is unknown, but it is usually underestimated hemipelvectomy (THP) was described frst by Turnbull in [3]. -
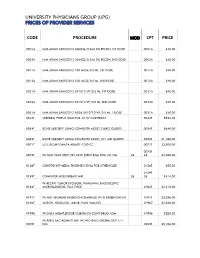
Code Procedure Cpt Price University Physicians Group
UNIVERSITY PHYSICIANS GROUP (UPG) PRICES OF PROVIDER SERVICES CODE PROCEDURE MOD CPT PRICE 0001A IMM ADMN SARSCOV2 30MCG/0.3ML DIL RECON 1ST DOSE 0001A $40.00 0002A IMM ADMN SARSCOV2 30MCG/0.3ML DIL RECON 2ND DOSE 0002A $40.00 0011A IMM ADMN SARSCOV2 100 MCG/0.5 ML 1ST DOSE 0011A $40.00 0012A IMM ADMN SARSCOV2 100 MCG/0.5 ML 2ND DOSE 0012A $40.00 0021A IMM ADMN SARSCOV2 5X1010 VP/0.5 ML 1ST DOSE 0021A $40.00 0022A IMM ADMN SARSCOV2 5X1010 VP/0.5 ML 2ND DOSE 0022A $40.00 0031A IMM ADMN SARSCOV2 AD26 5X10^10 VP/0.5 ML 1 DOSE 0031A $40.00 0042T CEREBRAL PERFUS ANALYSIS, CT W/CONTRAST 0042T $954.00 0054T BONE SURGERY USING COMPUTER ASSIST, FLURO GUIDED 0054T $640.00 0055T BONE SURGERY USING COMPUTER ASSIST, CT/ MRI GUIDED 0055T $1,188.00 0071T U/S LEIOMYOMATA ABLATE <200 CC 0071T $2,500.00 0075T 0075T PR TCAT PLMT XTRC VRT CRTD STENT RS&I PRQ 1ST VSL 26 26 $2,208.00 0126T CAROTID INT-MEDIA THICKNESS EVAL FOR ATHERSCLER 0126T $55.00 0159T 0159T COMPUTER AIDED BREAST MRI 26 26 $314.00 PR RECTAL TUMOR EXCISION, TRANSANAL ENDOSCOPIC 0184T MICROSURGICAL, FULL THICK 0184T $2,315.00 0191T PR ANT SEGMENT INSERTION DRAINAGE W/O RESERVOIR INT 0191T $2,396.00 01967 ANESTH, NEURAXIAL LABOR, PLAN VAG DEL 01967 $2,500.00 01996 PR DAILY MGMT,EPIDUR/SUBARACH CONT DRUG ADM 01996 $285.00 PR PERQ SAC AGMNTJ UNI W/WO BALO/MCHNL DEV 1/> 0200T NDL 0200T $5,106.00 PR PERQ SAC AGMNTJ BI W/WO BALO/MCHNL DEV 2/> 0201T NDLS 0201T $9,446.00 PR INJECT PLATELET RICH PLASMA W/IMG 0232T HARVEST/PREPARATOIN 0232T $1,509.00 0234T PR TRANSLUMINAL PERIPHERAL ATHERECTOMY, RENAL -

Slide Courtesy of Jeff North, MD
3/17/2017 Basic Dermatology Procedures Basic Dermatology Procedures for the Non‐dermatologist • Liquid Nitrogen • Skin Biopsies Lindy P. Fox, MD • Electrocautery Associate Professor Director, Hospital Consultation Service Department of Dermatology University of California, San Francisco [email protected] I have no conflicts of interest to disclose 1 Liquid Nitrogen Cryosurgery 1 3/17/2017 Liquid Nitrogen Cryosurgery Liquid Nitrogen Cryosurgery Principles • Indications • ‐ 196°C (−320.8°F) – Benign, premalignant, in situ malignant lesions • Temperatures of −25°C to −50°C (−13°F to −58°F) within 30 seconds with spray or probe • Objective – Selective tissue necrosis • Benign lesions: −20°C to −30°C (−4°F to −22°F) • Reactions predictable • Malignant lesions: −40°C to −50°C. – Crust, bulla, exudate, edema, sloughing • Post procedure hypopigmentation • Rapid cooling intracellular ice crystals • Slow thawing tissue damage – Melanocytes are more sensitive to freezing than • Duration of THAW (not freeze) time is most keratinocytes important factor in determining success Am Fam Physician. 2004 May 15;69(10):2365‐2372 Liquid Nitrogen Cryosurgery • Fast freeze, slow thaw cycles – Times vary per condition (longer for deeper lesion) – One cycle for benign, premalignant – Two cycles for warts, malignant (not commonly done) • Lateral spread of freeze (indicates depth of freeze) – Benign lesions 1‐2mm beyond margins – Actinic keratoses‐ 2‐3mm beyond margins – Malignant‐ 3‐5+mm beyond margins (not commonly done) From: Bolognia, Jorizzo, and Schaffer. -

Cross-Sectional Imaging in the Evaluation of Osteogenic Sarcoma: MRI and CT
Cross-sectional Imaging in the Evaluation of Osteogenic Sarcoma: MRI and CT By Leanne L. Seeger, Jeffrey J. Eckardt, and Lawrence W. Bassett EFORE the advent of cross-sectional scan- for treatment. Magnetic resonance imaging and B ning techniques, imaging of malignant bone CT image acquisition should address specific tumors was confined to plain radiography and issues that are important to the surgeon and radionuclide bone scanning. While radiography should be tailored according to tumor location remains the primary imaging modality for differ- and proposed surgical treatment, whether it be ential diagnosis, magnetic resonance imaging amputation or resection with limb salvage proce- (MRI) and computed tomography (CT) have dure. had a profound impact on the preoperative stag- The following discussion reflects the scanning ing evaluation of bone tumors and their response principles and techniques we use for evaluating to therapy. patients with OGS both preoperatively and post- In this article we will define the role of MRI operatively. This was derived from both our own and CT in the work-up of the patient with known experience and the experience of others as re- osteogenic sarcoma (OGS), stressing imaging ported in the literature. ‘-’ Regional differences in strategies that optimize information available to the management of patients with malignant bone the clinician and assist in therapy planning. In tumors may alter this procedure. To become order to achieve these optimal factors, communi- familiar with local treatment protocols, commu- cation with the referring physician and review of nicate directly with the surgeon who will ulti- available radiographs and radionuclide bone scans mately be responsible for treatment. -

ICD-9-CM Procedures (FY10)
2 PREFACE This sixth edition of the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) is being published by the United States Government in recognition of its responsibility to promulgate this classification throughout the United States for morbidity coding. The International Classification of Diseases, 9th Revision, published by the World Health Organization (WHO) is the foundation of the ICD-9-CM and continues to be the classification employed in cause-of-death coding in the United States. The ICD-9-CM is completely comparable with the ICD-9. The WHO Collaborating Center for Classification of Diseases in North America serves as liaison between the international obligations for comparable classifications and the national health data needs of the United States. The ICD-9-CM is recommended for use in all clinical settings but is required for reporting diagnoses and diseases to all U.S. Public Health Service and the Centers for Medicare & Medicaid Services (formerly the Health Care Financing Administration) programs. Guidance in the use of this classification can be found in the section "Guidance in the Use of ICD-9-CM." ICD-9-CM extensions, interpretations, modifications, addenda, or errata other than those approved by the U.S. Public Health Service and the Centers for Medicare & Medicaid Services are not to be considered official and should not be utilized. Continuous maintenance of the ICD-9- CM is the responsibility of the Federal Government. However, because the ICD-9-CM represents the best in contemporary thinking of clinicians, nosologists, epidemiologists, and statisticians from both public and private sectors, no future modifications will be considered without extensive advice from the appropriate representatives of all major users. -

SKIN GRAFTS and SKIN SUBSTITUTES James F Thornton MD
SKIN GRAFTS AND SKIN SUBSTITUTES James F Thornton MD HISTORY OF SKIN GRAFTS ANATOMY Ratner1 and Hauben and colleagues2 give excel- The character of the skin varies greatly among lent overviews of the history of skin grafting. The individuals, and within each person it varies with following highlights are excerpted from these two age, sun exposure, and area of the body. For the sources. first decade of life the skin is quite thin, but from Grafting of skin originated among the tilemaker age 10 to 35 it thickens progressively. At some caste in India approximately 3000 years ago.1 A point during the fourth decade the thickening stops common practice then was to punish a thief or and the skin once again begins to decrease in sub- adulterer by amputating the nose, and surgeons of stance. From that time until the person dies there is their day took free grafts from the gluteal area to gradual thinning of dermis, decreased skin elastic- repair the deformity. From this modest beginning, ity, and progressive loss of sebaceous gland con- skin grafting evolved into one of the basic clinical tent. tools in plastic surgery. The skin also varies greatly with body area. Skin In 1804 an Italian surgeon named Boronio suc- from the eyelid, postauricular and supraclavicular cessfully autografted a full-thickness skin graft on a areas, medial thigh, and upper extremity is thin, sheep. Sir Astley Cooper grafted a full-thickness whereas skin from the back, buttocks, palms of the piece of skin from a man’s amputated thumb onto hands and soles of the feet is much thicker.