SKIN GRAFTS and SKIN SUBSTITUTES James F Thornton MD
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Medical Policy
Medical Policy Joint Medical Policies are a source for BCBSM and BCN medical policy information only. These documents are not to be used to determine benefits or reimbursement. Please reference the appropriate certificate or contract for benefit information. This policy may be updated and is therefore subject to change. *Current Policy Effective Date: 5/1/21 (See policy history boxes for previous effective dates) Title: Composite Tissue Allotransplantation Description/Background Composite tissue allotransplantation refers to the transplantation of histologically different tissue that may include skin, connective tissue, blood vessels, muscle, bone, and nerve tissue. The procedure is also known as reconstructive transplantation. To date, primary applications of this type of transplantation have been of the hand and face (partial and full), although there are also reported cases of several other composite tissue allotransplantations, including that of the larynx, knee, and abdominal wall. The first successful partial face transplant was performed in France in 2005, and the first complete facial transplant was performed in Spain in 2010. In the United States, the first facial transplant was done in 2008 at the Cleveland Clinic; this was a near-total face transplant and included the midface, nose, and bone. The first hand transplant with short-term success occurred in 1998 in France. However, the patient failed to follow the immunosuppressive regimen, which led to graft failure and removal of the hand 29 months after transplantation. The -
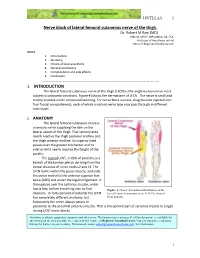
Nerve Block of Lateral Femoral Cutaneous Nerve of the Thigh
18VTLLAA 1 Nerve block of lateral femoral cutaneous nerve of the thigh. Dr. Robert M Raw (MD) . MBChB, MFGP, MPraxMed, DA, FCA. Professor of Anesthesia retired Editor of Regional-Anesthesia.Com INDEX. 1. Introduction 2. Anatomy 3. Choice of local anesthetic 4. General indications 5. Complications and side effects 6. Conclusion ------------------------------------------------------------------------------------ 1. INTRODUCTION The lateral femoral cutaneous nerve of the thigh (LFCN) is the single human nerve most subject to anatomic variations. Figure #1shows the dermatome of LFCN. The nerve is small and mostly invisible under ultrasound scanning. For nerve block success, drug must be injected into four fascial compartments, each of which a variant nerve type may pass through in different individuals. 2. ANATOMY The lateral femoral cutaneous nerve is a sensory nerve supplying the skin on the lateral aspect of the thigh. That sensory area nearly reaches the thigh posterior midline and the thigh anterior midline. Its superior limit passes over the greater trochanter and its inferior limit nearly reaches the height of the patella. The typical LCNT, in 60% of patients, is a branch of the lumbar plexus deriving from the dorsal divisions of nerve roots L2 and L3. The LFCN forms within the psoas muscle, and exits the pelvis medial to the anterior superior iliac spine (ASIS) and under the inguinal ligament. It then passes over the sartorius muscle, under fascia lata, before branching into its final Figure 1. Classic dermatomal distribution of the divisions. In forty percent of patients the LFCN lateral femoral cutaneous nerve (LFCN), derived has completely different anatomy, but from Sobotta. fortunately the nerve always passes in proximity to the proximal sartorius muscle. -

CRU DANCE Friday March 12Th - Sunday March 14Th, 2021 MASON, OH
CRU DANCE Friday March 12th - Sunday March 14th, 2021 MASON, OH th Friday March 12 , 2021 Start Time: 5:00pm Studio Check in- Studio B, F, Q 4:30 PM Jadden Hahn, Shaylee Knott, Heidi Schleidt, 1 Q Coffee Anyone? Teen Tap Small Group Intermediate 5:00 PM Eliana Tipton Anna Armold, Olivia Ashmore, Lana Baker, Gabrielle Barbosa, Lilliana Barbosa, Quinnlyn Musical Blaisdell, Annabel Blake, Ava Constable, 2 F Little Girls Junior Large Group Novice 5:03 PM Theater Morgan Flick, Bella Hughes, Madelyn Hughes, Abigail Larson, Teagan Manley, Andromeda Search, Jackie Wright, Pammy Wright 3 Q I Can Be Anything Petite Jazz Solo Novice Arianna Liggett 5:07 PM 4 Q Miss Invisible Petite Lyrical Solo Novice Makenzie Berling 5:09 PM Piper Hays, Brooklyn Homan, Franky Jellison, 5 B Booty Swing Junior Jazz Small Group Intermediate Janessa Smuts, Sophie Steinbrunner, 5:12 PM Madison Wendeln 6 F Meant Senior Contemporary Solo Intermediate Pammy Wright 5:15 PM 7 B Womanizer Senior Jazz Solo Intermediate Shelby Ranly 5:18 PM 8 Q Just Mime'en My Business Junior Acrobat Solo Novice Alexis Burke 5:21 PM I Just Died in Your Arms 9 F Junior Open Solo Elite Jackie Wright 5:24 PM Tonight Anna Armold, Quinnlyn Blaisdell, Morgan 10 F Shadows of the Night Junior Jazz Small Group Intermediate 5:26 PM Flick, Madelyn Hughes Jadden Hahn, Gabrielle Helton, Shaylee 11 Q Amazing Grace Teen Contemporary Small Group Intermediate Knott, Reese Lynch, Heidi Schleidt, Eliana 5:29 PM Tipton 12 F Speechless Mini Jazz Solo Novice Teagan Manley 5:32 PM 13 B Dynamite Junior Jazz Solo Intermediate -

CPT® New Codes 2019: Biopsy, Skin
Billing and Coding Update Alexander Miller, M.D. AAD Representative to the AMA CPT Advisory Committee New Skin Biopsy CPT® Codes It’s all about the Technique! SPEAKER: Alexander Miller, M.D. AAD Representative to the AMA -CPT Advisory Committee Chair AAD Health Care Finance Committee Arriving on January 1, 2019 New and Restructured Biopsy Codes Tangential biopsy Punch Biopsy Incisional Biopsy How Did We Get Here? CMS CY 2016 Biopsy codes (11100, 11101 identified as potentially mis-valued; high expenditure RUC Survey sent to AAD Members Specialty survey results are the only tool available to support code values Challenging survey results Survey revealed bimodal data distribution; CPT Codes 11100, 11101 referred to CPT for respondents were valuing different procedures restructuring Rationale for New Codes 11100; 11101 • Previous skin biopsy codes did not distinguish between the different biopsy techniques that were being used CPT Recommended technique specification in new biopsy codes • Will also provide for reimbursement commensurate with the technique used How Did We Get Here? • CPT Editorial Panel deleted 11100; 11101 February 2017 • 6 New codes created based on technique utilized • Each technique: primary code and add-on code March 2017 • RUC survey sent to AAD members April 2017 • Survey results presented to the RUC Biopsy Codes Effective Jan., 1, 2019 • Integumentary biopsy codes 11755 Biopsy of nail unit (plate, bed, matrix, hyponychium, proximal and lateral nail folds 11100, 11101 have been deleted 30100 Biopsy, intranasal • New -

Optimizing Breast Reconstruction After Mastectomy University of Antwerp Faculty of Medicine and Health Sciences
Optimizing breast reconstruction after mastectomy mastectomy after reconstruction Optimizing breast Filip Thiessen University of Antwerp Faculty of Medicine and Health Sciences Optimizing breast reconstruction after mastectomy The use of dynamic infrared thermography Filip THIESSEN 2020 Antwerp, 2020 Thesis submitted in fulfilment of Promoters: Prof. dr. Wiebren Tjalma the requirements for the degree of Prof. dr. Gunther Steenackers Doctor in Medical Sciences at the Prof. dr. Guy Hubens University of Antwerp Co-promoter: Prof. dr. Veronique Verhoeven University of Antwerp Faculty of Medicine and Health Sciences Optimizing breast reconstruction after mastectomy: The use of dynamic infrared thermography Optimaliseren van borstreconstructies na mastectomie: Het gebruik van dynamic infrared thermography Thesis submitted in fulfilment of the requirements for the degree of Doctor in Medical Sciences at the University of Antwerp to be defended by Filip THIESSEN Proefschrift voorgelegd tot het behalen van de graad van doctor in de Medische Wetenschappen aan de Universiteit Antwerpen te verdedigen door Antwerpen, 2020 Promotoren: Prof. dr. Wiebren Tjalma Prof. dr. Gunther Steenackers Prof. dr. Guy Hubens Begeleider: Prof. dr. Veronique Verhoeven Promotoren Prof. dr. Wiebren Tjalma Prof. dr. Gunther Steenackers Prof. dr. Guy Hubens Begeleider Prof. dr. Veronique Verhoeven Members of the jury Internal Prof. dr. Jeroen Hendriks Prof. dr. Manon Huizing Prof. dr. Wiebren Tjalma Prof. dr. Gunther Steenackers Prof. dr. Guy Hubens External Prof. dr. Emiel Rutgers Prof. dr. Assaf Zeltzer © Filip Thiessen Optimizing breast reconstruction after mastectomy: The use of dynamic infrared thermography / Filip Thiessen Faculteit Geneeskunde, Universiteit Antwerpen, Antwerpen 2020 Thesis Universiteit Antwerpen – with summary in Dutch Lay-out and cover : Dirk De Weerdt (www.ddwdesign.be) Cover figure: Cold challenge to bilateral DIEP in skin sparing mastectomy (top), rapid and overall rewarming of the skin islands of the DIEP flap (bottom). -

Transplantation and Hepatic Pathology University of Pittsburgh Medical Center November, 2007
Resident Handbook Division of Transplantation and Hepatic Pathology University of Pittsburgh Medical Center November, 2007 For private use of residents only- not for public distribution Table of Contents Anatomic Transplantation Pathology Rotation Clinical Responsibilities of the Division ........................................................3 Categorizations of Specimens and Structure of Signout.................................3 Resident Responsibilities................................................................................4 Learning Resources.........................................................................................4 Transplantation Pathology on the World-Wide Web......................................4 Weekly Schedule ............................................................................................6 Staff Locations and Telephone Numbers........................................................7 Background Articles Landmarks in Transplantation ........................................................................8 Trends in Organ Donation and Transplantation US 1996-2005.....................18 Perspectives in Organ Preservation………....................................................26 Transplant Tolerance- Editorial……………………………………….…….36 Kidney Grading Systems Banff 2005 Update……………………….....................................................42 Banff 97 Components (I t v g etc.) ................................................................44 Readings Banff 05 Meeting Report………………………………………...................47 -

Thin Anterolateral Thigh Perforator Flaps: Minimizing the Partial Necrosis Rate
Extremity/Lymphedema Original Article The perforator-centralizing technique for super- thin anterolateral thigh perforator flaps: Minimizing the partial necrosis rate Young Chul Suh, Na Rim Kim, Dai Won Jun, Jung Ho Lee, Young Jin Kim Department of Plastic and Reconstructive Surgery, Bucheon St. Mary Hospital, College of Medicine, The Catholic University of Korea, Bucheon, Korea Background Despite the wide demand for thin flaps for various types of extremity recon- Correspondence: Young Chul Suh struction, the thin elevation technique for anterolateral thigh (ALT) flaps is not very popular Department of Plastic and Reconstructive Surgery, Bucheon St. because of its technical difficulty and safety concerns. This study proposes a novel perforator- Mary Hospital, College of Medicine, centralizing technique for super-thin ALT flaps and analyzes its effects in comparison with a The Catholic University of Korea, 327 skewed-perforator group. Sosa-ro, Wonmi-gu, Bucheon 14647, Methods From June 2018 to January 2020, 41 patients who required coverage of various Korea Tel: +82-32-340-2095 types of defects with a single perforator-based super-thin ALT free flap were enrolled. The in- Fax: +82-32-340-7227 cidence of partial necrosis and proportion of the necrotic area were analyzed on postopera- E-mail: [email protected] tive day 20 according to the location of superficial penetrating perforators along the flap. The centralized-perforator group was defined as having a perforator anchored to the middle third of the x- and y-axes of the flap, while the skewed-perforator group was defined as having a perforator anchored outside of the middle third of the x- and y-axes of the flap. -

Burn Contracture Surgery
Burn Contracture Surgery Stuart Watson Canniesburn Unit Glasgow Royal Infirmary Glasgow United Kingdom [email protected] 1 Table of Contents Prevention of Contractures ................................................................................................. 4 Contracture Definitions ....................................................................................................... 4 Timing of Contracture Surgery: Indications ....................................................................... 5 Urgent ............................................................................................................................. 5 Early ................................................................................................................................ 5 Late ................................................................................................................................. 5 General Principles and Technical Tips ............................................................................... 6 Approach to Contracture Surgery ....................................................................................... 8 Split Skin Grafts .................................................................................................................. 8 Full Thickness Grafts .......................................................................................................... 9 Dermal Substitutes ............................................................................................................. -

Msc Payment Schedule Index
MSC PAYMENT SCHEDULE INDEX SECTIONS (To go directly to the an applicable section of the Payment Schedule, click on the Section heading listed below) GENERAL PREAMBLE TO THE PAYMENT SCHEDULE .................................. 1 2. OUT-OF-OFFICE HOURS PREMIUMS ............................................................... 2 3. GENERAL SERVICES ......................................................................................... 3 4. DIAGNOSTIC AND SELECTED THERAPEUTIC PROCEDURES ...................... 4 5. CRITICAL CARE .................................................................................................. 5 6. EMERGENCY MEDICINE .................................................................................... 6 7. GENERAL PRACTICE ......................................................................................... 7 8. ANESTHESIA ...................................................................................................... 8 9. DERMATOLOGY ................................................................................................. 9 10. OPHTHALMOLOGY .......................................................................................... 10 11. OTOLARYNGOLOGY ........................................................................................ 11 12. GENERAL INTERNAL MEDICINE .................................................................... 12 13. CARDIOLOGY ................................................................................................... 13 14. CLINICAL IMMUNOLOGY AND ALLERGY -

Costal Cartilage Graft in Asian Rhinoplasty: Surgical Techniques Sarina Rajbhandari and Chuan-Hsiang Kao
Chapter Costal Cartilage Graft in Asian Rhinoplasty: Surgical Techniques Sarina Rajbhandari and Chuan-Hsiang Kao Abstract Asian rhinoplasty is one of the most difficult and challenging surgeries in facial plastic surgery. As many Asians desire a higher nasal bridge and a refined nasal tip, they undergo various augmentation procedures such as artificial implant grafting and filler injections. Autologous rib graft is a very versatile graft material that can be used to augment the nose, with lesser complications, if done precisely. In this chapter, we have discussed the steps of rib graft harvesting, carving and setting into the nose to form a new dorsal height. Keywords: rhinoplasty, costal cartilage, Asians, warping 1. Introduction Asian rhinoplasty is one of the most difficult and challenging surgeries in facial plastic surgery. In Asians, the most common complaints regarding appearance of the nose are a low dorsum and an unrefined tip. Thus, most Asian rhinoplasties include augmentation of the nasal dorsum using either autologous or artificial implant, and/or nasal tip surgery. Clients who have had augmentation rhinoplasty previously frequently opt for revision. Hence, when a client comes for augmenta- tion or Asian rhinoplasty, the surgeon has to confirm whether the client has had any rhinoplasty (or several rhinoplasties) earlier. Artificial nasal implants for augmentation are still in vogue, owing to their simplicity and efficiency, but they are accompanied by several major and minor complications. Revision surgeries for these complications include correcting nasal contour deformities and fix functional problems, and require a considerable amount of cartilage. Revision surgeries are more complex than primary Asian rhinoplasty as they require intricate reconstruc- tion and the framework might be deficient. -

Preparatory: 1 Venous Access and Medication Administration: 4
Preparatory: 1 Venous Access and Medication Administration: 4 W4444444444444444444444444444444444444444444444444444444444444444444444444444444444444444444444444444444444444 UNIT TERMINAL OBJECTIVE 1-4 At the completion of this unit, the EMT-Critical Care Technician student will be able to safely and precisely access the venous circulation and administer medications. COGNITIVE OBJECTIVES At the completion of this unit, the EMT-Critical Care Technician student will be able to: 1-4.1 Review the specific anatomy and physiology pertinent to medication administration. (C-1) 1-4.2 Review mathematical principles. (C-1) 1-4.3 Review mathematical equivalents. (C-1) 1-4.4 Differentiate temperature readings between the Centigrade and Fahrenheit scales. (C-3) 1-4.5 Discuss formulas as a basis for performing drug calculations. (C-1) 1-4.6 Calculate oral and parenteral drug dosages for all emergency medications administered to adults, infants and children. (C-2) 1-4.7 Calculate intravenous infusion rates for adults, infants, and children. (C-2) 1-4.8 Discuss legal aspects affecting medication administration. (C-1) 1-4.9 Discuss the "six rights" of drug administration and correlate these with the principles of medication administration. (C-1) 1-4.10 Discuss medical asepsis and the differences between clean and sterile techniques. (C-1) 1-4.11 Describe use of antiseptics and disinfectants. (C-1) 1-4.12 Describe the use of universal precautions and body substance isolation (BSI) procedures when administering a medication. (C-1) 1-4.13 Describe the indications, equipment needed, techniques utilized, precautions, and general principles of peripheral venous cannulation (Including saline locks). (C-1) 1-4.14 Describe the indications, equipment needed, techniques utilized, precautions, and general principles of intraosseous needle placement and infusion. -

Skins Uk Download Season 1 Episode 1: Frankie
skins uk download season 1 Episode 1: Frankie. Howard Jones - New Song Scene: Frankie in her room animating Strange Boys - You Can't Only Love When You Want Scene: Frankie turns up at college with a new look Aeroplane - We Cant Fly Scene: Frankie decides to go to the party anyway. Fergie - Glamorous Scene: Music playing from inside the club. Blondie - Heart of Glass Scene: Frankie tries to appeal to Grace and Liv but Mini chucks her out, then she gets kidnapped by Alo & Rich. British Sea Power - Waving Flags Scene: At the swimming pool. Skins Series 1 Complete Skins Series 2 Complete Skins Series 3 Complete Skins Series 4 Complete Skins Series 5 Complete Skins Series 6 Complete Skins - Effy's Favourite Moments Skins: The Novel. Watch Skins. Skins in an award-winning British teen drama that originally aired in January of 2007 and continues to run new seasons today. This show follows the lives of teenage friends that are living in Bristol, South West England. There are many controversial story lines that set this television show apart from others of it's kind. The cast is replaced every two seasons to bring viewers brand new story lines with entertaining and unique characters. The first generation of Skins follows teens Tony, Sid, Michelle, Chris, Cassie, Jal, Maxxie and Anwar. Tony is one of the most popular boys in sixth form and can be quite manipulative and sarcastic. Michelle is Tony's girlfriend, who works hard at her studies, is very mature, but always puts up with Tony's behavior.