EASD2020 Finalprogramme.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Exenatide QW: a New Treatment Option for Type 2 Diabetes Offering Ease of Use, Improved Efficacy, and Reduced Side Effects
FEATURE ARTICLE Commentary: Exenatide QW: A New Treatment Option for Type 2 Diabetes Offering Ease of Use, Improved Efficacy, and Reduced Side Effects Charles F. Shaefer, Jr., MD Editor’s note: Once-weekly exenatide, (also called incretin mimetics) cur- with GLP-1 receptor agonists is the which has recently been approved for rently in use.3,4 only form of anti-diabetes therapy patients with type 2 diabetes, has great Validation of the acceptance offering proven weight loss. Finally, potential as a new diabetes therapy in clinical practice of GLP-1 GLP-1 receptor agonist therapy is in the primary care setting. This receptor agonists can be found in one of the recommended treatment commentary and the feature article that the recently released American strategies offering a low incidence of follows it (p. 95) offer an overview of Diabetes Association (ADA)/ hypoglycemia, an important aspect this new therapeutic tool and important European Association for the Study of diabetes therapy learned from the insights about its clinical utility. In the of Diabetes (EASD) position state- Action to Control Cardiovascular interest of transparency, however, we ment on the management of type 2 Risk in Diabetes trial.6 want to point out that the authors of diabetes, which placed these drugs As clinicians consider what to do both articles are affiliated with Amylin alongside older, well-accepted agents when metformin and lifestyle change Pharmaceuticals, which manufactures such as sulfonylureas (SUs) and do not adequately control a patient’s exenatide QW and markets it under the thiozolidinediones (TZDs) as a sec- A1C, they frequently ask, “incretin 5 trade name Bydureon. -

GLP-1 Receptor Agonists
Cognitive Vitality Reports® are reports written by neuroscientists at the Alzheimer’s Drug Discovery Foundation (ADDF). These scientific reports include analysis of drugs, drugs-in- development, drug targets, supplements, nutraceuticals, food/drink, non-pharmacologic interventions, and risk factors. Neuroscientists evaluate the potential benefit (or harm) for brain health, as well as for age-related health concerns that can affect brain health (e.g., cardiovascular diseases, cancers, diabetes/metabolic syndrome). In addition, these reports include evaluation of safety data, from clinical trials if available, and from preclinical models. GLP-1 Receptor Agonists Evidence Summary GLP-1 agonists are beneficial for patients with type 2 diabetes and obesity. Some evidence suggests benefits for Alzheimer’s disease. It is unclear whether it is beneficial for individuals without underlying metabolic disease. Semaglutide seems to be most effective for metabolic dysfunction, though liraglutide has more preclinical data for Alzheimer’s disease. Neuroprotective Benefit: Evidence from many preclinical studies and a pilot biomarker study suggest some neuroprotective benefits with GLP-1 agonists. However, whether they may be beneficial for everyone or only a subset of individuals (e.g. diabetics) is unclear. Aging and related health concerns: GLP-1 agonists are beneficial for treating diabetes and cardiovascular complications relating to diabetes. It is not clear whether they have beneficial effects in otherwise healthy individuals. Safety: GLP-1 agonists are generally safe for most people with minor side effects. However, long-term side effects are not known. 1 Availability: Available Dose: Varies - see Chemical formula: C172H265N43O51 (Liraglutide) as a prescription chart at the end of MW: 3751.262 g/mol medicine. -

Multifaceted Physiological Roles of Adiponectin in Inflammation And
International Journal of Molecular Sciences Review Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases Hyung Muk Choi 1, Hari Madhuri Doss 1,2 and Kyoung Soo Kim 1,2,* 1 Department of Clinical Pharmacology and Therapeutics, Kyung Hee University School of Medicine, Seoul 02447, Korea; [email protected] (H.M.C.); [email protected] (H.M.D.) 2 East-West Bone & Joint Disease Research Institute, Kyung Hee University Hospital at Gangdong, Gandong-gu, Seoul 02447, Korea * Correspondence: [email protected]; Tel.: +82-2-961-9619 Received: 3 January 2020; Accepted: 10 February 2020; Published: 12 February 2020 Abstract: Adiponectin is the richest adipokine in human plasma, and it is mainly secreted from white adipose tissue. Adiponectin circulates in blood as high-molecular, middle-molecular, and low-molecular weight isoforms. Numerous studies have demonstrated its insulin-sensitizing, anti-atherogenic, and anti-inflammatory effects. Additionally, decreased serum levels of adiponectin is associated with chronic inflammation of metabolic disorders including Type 2 diabetes, obesity, and atherosclerosis. However, recent studies showed that adiponectin could have pro-inflammatory roles in patients with autoimmune diseases. In particular, its high serum level was positively associated with inflammation severity and pathological progression in rheumatoid arthritis, chronic kidney disease, and inflammatory bowel disease. Thus, adiponectin seems to have both pro-inflammatory and anti-inflammatory effects. This indirectly indicates that adiponectin has different physiological roles according to an isoform and effector tissue. Knowledge on the specific functions of isoforms would help develop potential anti-inflammatory therapeutics to target specific adiponectin isoforms against metabolic disorders and autoimmune diseases. -

Combining a Glucagon-Like Peptide-1 Receptor Agonist with Basal Insulin: the Why and How
Combining a Glucagon-like Peptide-1 Receptor Agonist with Basal Insulin: The Why and How Case Study Mary is a 61 year-old female diagnosed with type 2 diabetes mellitus (T2DM) 8 years ago. She was initially managed with the combination of lifestyle modification and metformin. Since that time she was treated with a sulfonylurea, but it was discontinued due to symptomatic hypoglycemia. She was also treated with pioglitazone, but significant fluid retention led to it discontinuation. A year-and-a- half ago, basal insulin was added to her lifestyle and metformin management. She now administers 52 units (0.62 units/kg) once daily at bedtime. Since starting basal insulin, she has experienced 3 episodes of mild hypoglycemia. Since her diagnosis, Mary’s HbA1c has never been <7.0%; her current HbA1c is 7.9%. Over the past month, her fasting plasma glucose (FPG) has ranged from 103 mg/dL to 136 mg/dL and her postprandial glucose (PPG) from 164 mg/dL to 213 mg/dL. She has gained 2.6 kg since starting basal insulin and her body mass index is now 31 kg/m2. Her blood pressure is 134/82 mmHg. She experiences occasional tingling in her feet. Eye examination reveals grade 1 retinopathy. Current medications are: metformin 1000mg twice daily, basal insulin 52 units once daily at bedtime, and hydrochlorothiazide 25 mg once daily. Her family physician notes that Mary’s FPG is reasonably well-controlled, yet her HbA1c and PPG remain elevated. He is also concerned about her episodes of hypoglycemia and weight gain and the evidence indicating microvascular damage. -

Footprints an Informational Newsletter for Patients of APMA Member Podiatrists October 2017
footprints an informational newsletter for patients of APMA member podiatrists october 2017 special EDITION include a DPM for diabetes prevention & Manage Ment According to the CDC, more than 100 million US adults are living with either diabetes or prediabetes. If you have diabetes or prediabetes, it is essential to include a podiatrist for proper diabetes prevention and management. In fact, podiatrists can reduce amputation rates up to 80 percent. In order for you not to become a part of that statistic, here are a few simple things you can do to keep your risk for diabetic ulcers and amputation low: Inspect feet daily. Check your Don’t go barefoot. Don’t go 1 feet and toes every day for 5 without shoes, even in your cuts, bruises, sores, or changes to own home. The risk of cuts and the toenails, such as thickening or infection is too great for those discoloration. with diabetes. Wear thick, soft socks. Avoid Never try to remove calluses, 2 socks with seams, which could 6 corns, or warts by yourself. rub and cause blisters or other Over-the-counter products can burn skin injuries. the skin and cause irreparable damage to the foot for people Exercise. Walking can keep with diabetes. 3 weight down and improve circulation. Be sure to wear See a podiatrist. Regular appropriate athletic shoes when 7 checkups by a podiatrist— exercising. at least annually—are the best WALKING CAN KEEP way to ensure that your feet WEIGHT DOWN AND Have new shoes properly remain healthy. 4 measured and fitted. Foot size IMPROVE CIRCULATION. -

Adiporon, an Orally Active, Synthetic Agonist of Adipor1 and Adipor2 Receptors Has Gastroprotective Effect in Experimentally Induced Gastric Ulcers in Mice
molecules Article AdipoRon, an Orally Active, Synthetic Agonist of AdipoR1 and AdipoR2 Receptors Has Gastroprotective Effect in Experimentally Induced Gastric Ulcers in Mice Hubert Zatorski 1,2, Maciej Salaga 1 , Marta Zieli ´nska 1, Kinga Majchrzak 1, Agata Binienda 1 , Radzisław Kordek 3, Ewa Małecka-Panas 2 and Jakub Fichna 1,4,* 1 Department of Biochemistry, Medical University of Lodz, 92-215 Lodz, Poland; [email protected] (H.Z.); [email protected] (M.S.); [email protected] (M.Z.); [email protected] (K.M.); [email protected] (A.B.) 2 Department of Digestive Tract Diseases, Medical University of Lodz, 93-281 Lodz, Poland; [email protected] 3 Department of Pathology, Medical University of Lodz, 92-215 Lodz, Poland; [email protected] 4 Department of Biochemistry, Faculty of Medicine, Medical University of Lodz, Mazowiecka 6/8, 92-215 Lodz, Poland * Correspondence: jakub.fi[email protected]; Tel.: +48-42-272-57-07 Citation: Zatorski, H.; Salaga, M.; Abstract: Introduction: Adiponectin is a hormone secreted by adipocytes, which exhibits insulin- Zieli´nska,M.; Majchrzak, K.; sensitizing and anti-inflammatory properties and acts through adiponectin receptors: AdipoR1 and Binienda, A.; Kordek, R.; AdipoR2. The aim of the study was to evaluate whether activation of adiponectin receptors AdipoR1 Małecka-Panas, E.; Fichna, J. and AdipoR2 with an orally active agonist AdipoRon has gastroprotective effect and to investigate AdipoRon, an Orally Active, the possible underlying mechanism. Methods: We used two well-established mouse models of Synthetic Agonist of AdipoR1 and gastric ulcer (GU) induced by oral administration of EtOH (80% solution in water) or diclofenac AdipoR2 Receptors Has (30 mg/kg, p.o.). -

Diabetic Foot Ulcers
REVIEW Review Diabetic foot ulcers William J Jeffcoate, Keith G Harding Ulceration of the foot in diabetes is common and disabling and frequently leads to amputation of the leg. Mortality is high and healed ulcers often recur. The pathogenesis of foot ulceration is complex, clinical presentation variable, and management requires early expert assessment. Interventions should be directed at infection, peripheral ischaemia, and abnormal pressure loading caused by peripheral neuropathy and limited joint mobility. Despite treatment, ulcers readily become chronic wounds. Diabetic foot ulcers have been neglected in health-care research and planning, and clinical practice is based more on opinion than scientific fact. Furthermore, the pathological processes are poorly understood and poorly taught and communication between the many specialties involved is disjointed and insensitive to the needs of patients. In this review, we describe the epidemiology, pathogenesis, operations above the ankle or those proximal to the and management of diabetic foot ulceration, and its effect tarsometatarsal joint, and a multinational WHO survey on on patients and society. The condition deserves more lower extremity amputation also included cases of attention, both from those who provide care and those who unoperated gangrene.13 fund research. Moreover, amputation is a marker not just of disease but also of disease management. The decision to operate Epidemiology is determined by many factors, which vary between Incidence and prevalence centres and patients. A high amputation rate might result Although accurate figures are difficult to obtain for the from high disease prevalence, late presentation, and prevalence or incidence of foot ulcers, the results of cross- inadequate resources, but could also reflect a particular sectional community surveys in the UK showed that 5·3% approach by local surgeons. -

Performance of the Insulin-Only Ilet Bionic Pancreas and The
e118 Diabetes Care Volume 44, June 2021 Performance of the Insulin-Only Luz E. Castellanos,1 Courtney A. Balliro,1 Jordan S. Sherwood,1 Rabab Jafri,1 iLet Bionic Pancreas and the Mallory A. Hillard,1 Evelyn Greaux,1 Rajendranath Selagamsetty,2 Hui Zheng,3 Bihormonal iLet Using Firas H. El-Khatib,2 Edward R. Damiano,2,4 and Dasiglucagon in Adults With Type Steven J. Russell1 1 Diabetes in a Home-Use Setting Diabetes Care 2021;44:e118–e120 | https://doi.org/10.2337/dc20-1086 Reductions in blood glucose levels in with insulin lispro (Eli Lilly) or aspart (Table 1). The mean CGM glucose and people with diabetes are often achieved (Novo Nordisk), the bihormonal iLet for time in range (70–180 mg/dL) were 149 at the expense of increased hypoglyce- 7dayswithdasiglucagon(4mg/mL) ±13mg/dLand72±8%,respectively,in mia. A novel approach is to automati- and insulin lispro or aspart, or both, us- the insulin-only period, and 139 ± 11 cally deliver microdose glucagon when ing the same glucose target (110 mg/ mg/dL and 79 ± 9%, respectively, in the automation of insulin delivery alone is dL), in random order. There were no re- bihormonal period. The mean daily car- not sufficient to prevent hypoglycemia. strictions on diet or exercise. The prima- bohydrates consumed to prevent or The approach requires a bihormonal de- ry outcomes were prespecified iLet treat hypoglycemia were 16 ± 13 g and vice and a stable form of glucagon or operational thresholds. The key second- 18 ± 21 g in the insulin-only and bihor- glucagon analog. -

(Pram) and Insulin A21G Improves Post-Prandial Glucose Vs Novolog
ADO09, A Co-Formulation Of Pramlintide (Pram) and Insulin A21G improves Post-Prandial Glucose Vs Novolog® in Type 1 Diabetes (T1DM) G.Meiffren¹, G.Andersen², R.Eloy¹, C.Seroussi¹, C.Mégret¹, S.Famulla², Y.-P Chan¹, M.Gaudier¹, O.Soula¹, J.H. DeVries²,T.Heise² (1 Adocia, Lyon, France ; 2 Profil, Neuss, Germany) Introduction & Background Overall safety Outpatient period results - CGM metrics o ADO09 (M1Pram) is a co-formulation of pramlintide and insulin A21G o Both treatments were well tolerated without any treatment-related serious adverse events o Most of the CGM metrics (TiR [70-180], TiR [80-140], mean blood glucose per day), were significantly improved developed to leverage the beneficial effects of pramlintide on post-prandial (Table 2). As expected M1Pram had numerically more, mostly gastrointestinal adverse events with M1Pram (Table 4). Postprandial and mean 24-hour glucose profiles were improved with M1Pram (Fig. 3) glucose without additional injections than insulin aspart Table 4: CGM metrics, all days. Significant differences are marked in bold Objective and design o No severe hypoglycemia were seen, slightly more hypoglycemic events occurred with M1Pram Ratio of LSMean* o To compare the effect of M1Pram and insulin aspart (Novolog®, Novo than with aspart (Table 3) Difference Parameter Treatment LS Mean M1Pram / Aspart P-value Nordisk) on post-prandial glucose control, glycemic control assessed by Table 2: Incidence of adverse events throughout the trial (M1Pram-Aspart) (95% CI) CGM and safety/tolerability M1Pram Aspart M1Pram -

TRULICITY, INN-Dulaglutide
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Trulicity 0.75 mg solution for injection in pre-filled pen Trulicity 1.5 mg solution for injection in pre-filled pen Trulicity 3 mg solution for injection in pre-filled pen Trulicity 4.5 mg solution for injection in pre-filled pen 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Trulicity 0.75 mg solution for injection in pre-filled pen Each pre-filled pen contains 0.75 mg of dulaglutide* in 0.5 ml solution. Trulicity 1.5 mg solution for injection in pre-filled pen Each pre-filled pen contains 1.5 mg of dulaglutide* in 0.5 ml solution. Trulicity 3 mg solution for injection in pre-filled pen Each pre-filled pen contains 3 mg of dulaglutide* in 0.5 ml solution. Trulicity 4.5 mg solution for injection in pre-filled pen Each pre-filled pen contains 4.5 mg of dulaglutide* in 0.5 ml solution. *produced in CHO cells by recombinant DNA technology. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Solution for injection. Clear, colourless solution. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Type 2 Diabetes Mellitus Trulicity is indicated for the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise • as monotherapy when metformin is considered inappropriate due to intolerance or contraindications • in addition to other medicinal products for the treatment of diabetes. For study results with respect to combinations, effects on glycaemic control and cardiovascular events, and the populations studied, see sections 4.4, 4.5 and 5.1. -
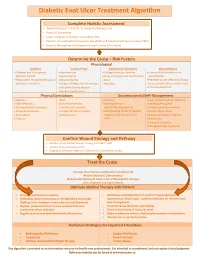
Diabetic Foot Ulcer Treatment Algorithm
Diabetic Foot Ulcer Treatment Algorithm Complete Holistic Assessment Medical/Surgical History & Co-morbidity Management Physical Examination Lower Leg (LLA) including monofilament test Perform Arterial Brachial Pressure Index (ABPI) and Toe Brachial Pressure Index (TBPI) Diabetic Management (Glycemic and Lipid Control & Nutrition) Determine the Cause – Risk Factors Physiological Diabetes Vascular Flow Autoimmune Disorders Wound History Diabetes and sub-optimal Hypertension Collagen vascular diseases History of foot infections or glycemic control Heart disease Immunosuppressant medications osteomyelitis Neuropathic changes with lack of Hyperlipidemia Gout Presence of toe infections (fungal protective sensation History of deep vein thrombosis Vasculitis or bacterial), callous and/or corns Peripheral artery disease Previous ulceration Venous Insufficiency Physical Limitations Socioeconomic/Self-Management Obesity Gait Smoking Lack of awareness for self-care Foot deformity Nutritional deficits Inadequate foot Inadequate hygiene Decreased level of activity Limited joint mobility wear/offloading devices Unsafe home environment Visual disturbances Congenital abnormalities Lack/Inability to afford diabetic Alcohol/drug abuse Amputation Osteoporosis supplies, foot care and foot Decreased Cognitive Ability Trauma wear Depression Financial insecurity Decreased level of activity Confirm Wound Etiology and Pathway Results of LLA, Monofilament Testing and ABPI/ TBPI Results of wound assessment Diagnostic -

Management of Hyperglycaemia and Steroid (Glucocorticoid) Therapy
Management of Hyperglycaemia and Steroid (Glucocorticoid) Therapy October 2014 This document is coded JBDS 08 in the series of JBDS documents Other JBDS documents: Admissions avoidance and diabetes: guidance for clinical commissioning groups and clinical team; December 2013, JBDS 07 The management of the hyperosmolar hyperglycaemic state (HHS) in adults with diabetes; August 2012, JBDS 06 Glycaemic management during the inpatient enteral feeding of stroke patients with diabetes; June 2012, JBDS 05 Self-management of diabetes in hospital; March 2012, JBDS 04 Management of adults with diabetes undergoing surgery and elective procedures: improving standards; April 2011, JBDS 03 The Management of Diabetic Ketoacidosis in Adults; revised September 2013, JBDS 02 The hospital management of hypoglycaemia in adults with diabetes mellitus; revised September 2013, JBDS 01 These documents are available to download from: ABCD website: www.diabetologists-abcd.org.uk/JBDS/JBDS.htm Diabetes UK website: www.diabetes.org.uk Contents Page Foreword 4 Authorship and acknowledgments 5-6 Introduction 7 Steroids - mechanism of action 8 Steroid therapy – impact on blood glucose 9 Glucose targets 10 Glucose monitoring 11 Diabetes treatment options 12-13 Treatment of steroid induced hyperglycaemia 14-15 Hospital discharge 16-17 Steroid treatment in pregnancy 18 Steroid treatment in end of life 19 Audit standards 20 Controversial areas 21 References 22 Appendix 1 – Algorithm to show treatment of steroid 23 induced diabetes Appendix 2 – Algorithm to show management of patients 24 with diabetes on once daily steroids Appendix 3 – End of life steroid management 25 Appendix 4 – Patient letter – Glucose monitoring and 26 steroid use 3 Foreword This is the latest in the series of Joint British Diabetes Societies for Inpatient Care (JBDS-IP) guidelines, and focuses on steroid induced hyperglycaemia and steroid induced diabetes.