Does the Growth Rate of Drifting Furcellaria Lumbricalis and Coccotylus Truncatus Depend on Their Proportion and Density?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hiiumaa 1 : 100
H I I U M A A 1 : 100 000 EESTI GEOLOOGILINE BAASKAART. RASKUSJÕUVÄLJA ANOMAALIAD GEOLOGICAL BASE MAP OF ESTONIA. GRAVITY ANOMALIES 5 0 5 0 5 0 5 0 5 0 5 0 5 0 8 9 9 0 0 1 1 2 2 3 3 4 4 5 22°0' 3 22°5' 3 22°10' 3 22°15' 4 22°20' 4 22°25' 4 22°30' 4 22°35' 4 22°40' 4 22°45' 4 22°50' 4 22°55' 4 23°0' 4 23°5' 4 23°10' LE GE N D 59°5' Lõimandi nina Isoanomaal -13,5 ,5 3 Isoanomal 6550 -13 5 6550 T a h k u-1n2, a 12 5 L e h t m a 59°5' - -11, -11 -10,5 Leh ,5 tm -9 a -10 j ,5 -8 5 , S -9 -7 ,5 u 14 11 8 5 2 -1 -4 -7 -10 -13 -16 mGal -6 u r M e e l s t e -8 ,5 j -5 ä M e e l s t e l a h t r -7 v -6 Kärrslätti neem K a u s t e VORMSI VARJUTATUD RELJEEF ("valgustatud" loodest) Kersli nina SHADED RELIEF ("lighting" from NW) 5 5 5 5 5 5 ORMSÖ 9 0 1 2 3 4 L Ä Ä N E M E R I 3 4 4 4 4 4 5 I 0, 6545 R Suursäär Kjulsnäs 6545 E (Kootsaare nina) M a n g u ,5 Kersleti jv -4 Tahkuna LKA M VORMSI Saxby neem E T a r e s t e l a h t Kjursskon K o d e s t e Tareste MKATõrvanina ORMSÖ -8 N ,5 M u d a s t e 6545 6545 Ä Kootsaare M a l v a s t e Ä poolsaar S i g a l a L T a r e s t e Vissulaid R i s t i 5,5 59°0' 6535 6535 2 Ninalaid R e i g i l a h t R e i g i R o o t s i K i d a s t e Vitberget K Ä R D L A 59°0' H a u s m a 5 , 6540 2 6540 K i r 6525 6525 i 5 5 k , , u ,5 2 4,5 5 5 l 2 5 Külalaid , a - 5, Paope LKA 5 h 6 Kadakalaid H - t P i h l a 5 , 4 Uuemererahu a P i l p a k ü l a 4 Elmrahu 5 4 Sääre nina - 6 Kukka laht r Kõrgessaare LKA K o i d m a Valgesäär i Västurvike KÕRGESSAARE 5 P a o p e l a h t T P , k (Västerviken) a ih 5 3 m l , K u k k a u 3 -

The Life Cycle and Genetic Structure of the Red Alga Furcellaria Lumbricalis on a Salinity Gradient
WALTER AND ANDRÉE DE NOTTBECK FOUNDATION SCIENTIFIC REPORTS View metadata, citation and similar papers at core.ac.uk brought to you by CORE No. 33 provided by Helsingin yliopiston digitaalinen arkisto The life cycle and genetic structure of the red alga Furcellaria lumbricalis on a salinity gradient KIRSI KOSTAMO Academic dissertation in Ecology, to be presented, with the permission of the Faculty of Biosciences of the University of Helsinki, for public criticism in the Auditorium 1041, Biocenter 2, University of Helsinki, Viikinkaari 5, Helsinki, on January 25th, 2008, at 12 noon. HELSINKI 2008 This thesis is based on the following papers, which will be referred to in the text by their Roman numerals: I. Kostamo, K. & Mäkinen, A. 2006: Observations on the mode and seasonality of reproduction in Furcellaria lumbricalis (Gigartinales, Rhodophyta) populations in the northern Baltic Sea. – Bot. Mar. 49: 304-309. II Korpelainen, H., Kostamo, K. & Virtanen, V. 2007: Microsatellite marker identifi cation using genome screening and restriction-ligation. – BioTechniques 42: 479-486. III Kostamo, K., Korpelainen, H., Maggs, C. A. & Provan, J. 2007: Genetic variation among populations of the red alga Furcellaria lumbricalis in northern Europe. – Manuscript. IV Kostamo, K. & Korpelainen, H. 2007: Clonality and small-scale genetic diversity within populations of the red alga Furcellaria lumbricalis (Rhodophyta) in Ireland and in the northern Baltic Sea. – Manuscript. Paper I is printed with permission from Walter de Gruyter Publishers and paper II with permission from BioTechniques. Reviewers: Dr. Elina Leskinen Department of Biological and Environmental Sciences University of Helsinki Finland Prof. Kerstin Johannesson Tjärnö Marine Biological Laboratory University of Gothenburg Sweden Opponent: Dr. -

Chemical Composition and Potential Practical Application of 15 Red Algal Species from the White Sea Coast (The Arctic Ocean)
molecules Article Chemical Composition and Potential Practical Application of 15 Red Algal Species from the White Sea Coast (the Arctic Ocean) Nikolay Yanshin 1, Aleksandra Kushnareva 2, Valeriia Lemesheva 1, Claudia Birkemeyer 3 and Elena Tarakhovskaya 1,4,* 1 Department of Plant Physiology and Biochemistry, Faculty of Biology, St. Petersburg State University, 199034 St. Petersburg, Russia; [email protected] (N.Y.); [email protected] (V.L.) 2 N. I. Vavilov Research Institute of Plant Industry, 190000 St. Petersburg, Russia; [email protected] 3 Faculty of Chemistry and Mineralogy, University of Leipzig, 04103 Leipzig, Germany; [email protected] 4 Vavilov Institute of General Genetics RAS, St. Petersburg Branch, 199034 St. Petersburg, Russia * Correspondence: [email protected] Abstract: Though numerous valuable compounds from red algae already experience high demand in medicine, nutrition, and different branches of industry, these organisms are still recognized as an underexploited resource. This study provides a comprehensive characterization of the chemical composition of 15 Arctic red algal species from the perspective of their practical relevance in medicine and the food industry. We show that several virtually unstudied species may be regarded as promis- ing sources of different valuable metabolites and minerals. Thus, several filamentous ceramialean algae (Ceramium virgatum, Polysiphonia stricta, Savoiea arctica) had total protein content of 20–32% of dry weight, which is comparable to or higher than that of already commercially exploited species Citation: Yanshin, N.; Kushnareva, (Palmaria palmata, Porphyra sp.). Moreover, ceramialean algae contained high amounts of pigments, A.; Lemesheva, V.; Birkemeyer, C.; macronutrients, and ascorbic acid. Euthora cristata (Gigartinales) accumulated free essential amino Tarakhovskaya, E. -

Download PDF Version
MarLIN Marine Information Network Information on the species and habitats around the coasts and sea of the British Isles A red seaweed (Furcellaria lumbricalis) MarLIN – Marine Life Information Network Biology and Sensitivity Key Information Review Will Rayment 2008-05-22 A report from: The Marine Life Information Network, Marine Biological Association of the United Kingdom. Please note. This MarESA report is a dated version of the online review. Please refer to the website for the most up-to-date version [https://www.marlin.ac.uk/species/detail/1616]. All terms and the MarESA methodology are outlined on the website (https://www.marlin.ac.uk) This review can be cited as: Rayment, W.J. 2008. Furcellaria lumbricalis A red seaweed. In Tyler-Walters H. and Hiscock K. (eds) Marine Life Information Network: Biology and Sensitivity Key Information Reviews, [on-line]. Plymouth: Marine Biological Association of the United Kingdom. DOI https://dx.doi.org/10.17031/marlinsp.1616.2 The information (TEXT ONLY) provided by the Marine Life Information Network (MarLIN) is licensed under a Creative Commons Attribution-Non-Commercial-Share Alike 2.0 UK: England & Wales License. Note that images and other media featured on this page are each governed by their own terms and conditions and they may or may not be available for reuse. Permissions beyond the scope of this license are available here. Based on a work at www.marlin.ac.uk (page left blank) Date: 2008-05-22 A red seaweed (Furcellaria lumbricalis) - Marine Life Information Network See online review for distribution map The seaweed Furcellaria lumbricalis, plant with fertile branches. -

Lõputöö-Hookan-Lember.Pdf (2.127Mb)
Sisekaitseakadeemia Päästekolledž Hookan Lember RS150 PÄÄSTESÜNDMUSTE ANALÜÜS PÜSIASUSTUSEGA VÄIKESAARTEL Lõputöö Juhendaja: Häli Allas MA Kaasjuhendaja: Andres Mumma Tallinn 2018 ANNOTATSIOON Päästekolledž Kaitsmine: juuni 2018 Töö pealkiri eesti keeles: Päästesündmuste analüüs püsiasustusega väikesaartel Töö pealkiri võõrkeeles: The analysis of rescue events on small islands with permanent settlements Lühikokkuvõte: Töö on kirjutatud eesti keeles ning eesti ja inglise keelse kokkuvõttega. Töö koos lisadega on kirjutatud kokku 61 lehel, millest põhiosa on 38 lehekülge. Lõputöö koosneb kolmest peatükist, kus on kasutatud kahte tabelit ja seitseteist joonist. Valitud teema uurimisprobleemiks on tervikliku ülevaate puudumine väikesaarte sündmustest, mis kuuluvad Päästeameti valdkonda. Väikesaartele toimub reageerimine erinevalt ning sõltuvalt aastaajast on reageerimine raskendatud. Ühtseid põhimõtteid rahvaarvu või sündmuste arvu kohta ei ole. Lõputöö eesmärk on analüüsida päästesündmusi väikesaartel aastatel 2009-2017 ning järeldada, millist päästevõimekust vajavad püsiasutustega väikesaared. Lõputöös antakse saartest ülevaade, mis on valimis välja toodud ning visualiseeritakse joonise abil saartel elavate püsielanike arv. Eesmärgi saavutamiseks kasutati kvantitatiivset uurimismeetodit, kus Häirekeskuselt saadud andmed korrastati ja analüüsiti. Lõputöös anti ülevaade, millised on sündmused saartel ning tehti järeldused, kuidas tagada kiire ja kvaliteetse abi kättesaadavus. Saartel, kus elanike arv on väike ning sündmuste arv minimaalne, -

Island Hopping in Estonia: a Hiiumaa, Saaremaa and Muhu Padise Tallinn Kardla Baltic Se Hiiumaa Haapsalu
SELF-GUIDED Etonia FINLAND Helsinki Island Hopping in Estonia: a Hiiumaa, Saaremaa and Muhu Padise Tallinn Kardla Baltic Se Hiiumaa Haapsalu ain/Bus Koguva Varbla Tr ESTONIA Leisi Pärnu Saaremaa Kuressaare LATVIA Tour distances: cycling ~480 km/300 mi, ferry 44 km, 12 days/11 nights TOUR INFORMATION Cycling grade: We grade this trip as easy to moder- ate. Daily signposted biking routes, mainly on roads with little traffic and cycle paths in towns. The terrain 12-days self guided cycling tour from/to Tallinn (Code: SG9P) is varied and rolling with some gradual hills on some cycling days between Tallinn and Padise. 3 ferries be- Estonia is characterised by its unique natural landscapes and traditional hospitality. Vibrant cities, tween the Estonian islands are planned on this tour desert beaches, peace and the unspoiled beauty of the countryside - a paradise for cyclists. But it’s the (pay locally). Estonian islands which are the jewels of the country. You’ll spend your holiday on these islands which Arrival & departure information / Transfers are amongst the most beautiful that the Baltic Sea has to offer. Lighthouses and windmills are the land- Airport: Tallinn (www.tallinn-airport.ee) marks of these islands. To begin your journey, you will get to know the Estonian capital Tallinn. This tour Ferry terminal: Tallinn (regular ferry lines from Hel- then takes you from Haapsalu to the second largest island Hiiumaa before crossing to the biggest island, sinki (FI) & Stockholm (SE)) Saaremaa. En route back to the mainland, you’ll cycle on the island of Muhu & from there into the sum- Transfer: (price for one way for up to 3 people) mer capital of Estonia, Pärnu. -

Hiiumaa Valla Beebid Kohtusid Suuremõisas
Sündisid uued vallakodanikud ELEANOR METSKÜLA ANDREAS VÕSA JOHANNES ARU Õnnitleme vanemaid! Juuni 2019, nr 18 Hiiumaa valla beebid kohtusid Suuremõisas FFoto:oto: AArgorgo NNursurs 10. mail kogunesid 30 pisikest tööspetsialistide meisterdatud vallakodanikku koos peredega kaardi. Sama kujundusega oli ka Suuremõisa Lossi, et tähistada pidupäevaks küpsetatud Hiiumaa Hiiumaa aasta ema 2019 on Ly Klee Hiiumaa valla beebipäeva. Köök ja Pagari tort. Külalised Ehkki kõik kutsutud väikelapsed kiitsid nii torti kui suupistelauda, Meie tänavuse aasta ema väga hinnatud spetsialist oma ei saanud tulla, oli kohal hulga mille kattis Aimar Hansen. peres kasvab viis last, kel- täpsuse ja töökuse poolest. Ette- emasid ja isasid, õdesid ja vendi Hiljem said kõik pered soovi kor- lest kaks on kasulapsed, paneku tegija on teda kirjeldanud ning terve lossisaal oli rahvast ral lasta fotograaf Argo Nursil aga armastust jagub neile kui tugevat ja hoolivat ema, kes täis. end pildile jäädvustada ja ühispilt vajadusel julgeb seista nii enda kõikidele ühtemoodi. kui ka teiste laste eest. Olles Väiksemaid ja suuremaid val- tehti lossitrepile. ühiskondlikult aktiivne ja samas lakodanikke tervitas Emmaste Palju sära ja rõõmu kõikidele uute Selle pere lapsed on kõik väga luua soe ning armastav kodu oma osavalla sotsiaaltööspetsialist vallakodanikele ning nende pere- tublid ja hakkajad ning kahel on abikaasale ning viiele lapsele on Liia Rull, kelle sõnavõtule järgnes dele ja toredate kohtumisteni! jalge all juba ülikoolitee. Ema märkimisväärne oskus! Külli Kreegi juhendamisel tore suutnud säilitada positiivse el- muusikaline ülesaste Suuremõisa lusuhtumise ning olla olemas Palju õnne, armas Ly! lasteaed-põhikooli õpilastelt. Liia kõikide laste jaoks, luues neile Aasta ema kuulutati välja reedel, luges vahva luuletuse, saatjaks koos abikaasaga toetav ja ühte- 10. -
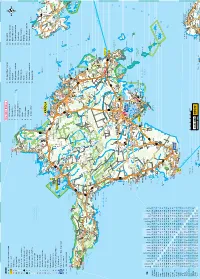
V Ä I N a M E
LEGEND Ring ümber Hiiumaa 9. Hiiumaa Militaarmuuseum 19. Kuriste kirik Turismiinfokeskus; turismiinfopunkt 1. Põlise leppe kivid 10. Mihkli talumuuseum 20. Elamuskeskus Tuuletorn Sadam; lennujaam Tahkuna nina Tahkuna 2. Pühalepa kirik 11. Reigi kirik 21. Käina kiriku varemed Haigla; apteek looduskaitseala 14 3. Suuremõisa loss 12. Kõrgessaare – Viskoosa 22. Orjaku linnuvaatlustorn Kirik; õigeusukirik Tahkuna 4. Soera talumuuseum ja Mõis; kalmistu 13. Kõpu tuletorn 23. Orjaku sadam kivimitemaja Huvitav hoone; linnuse varemed 14. Ristna tuletorn 24. Kassari muuseum 5. Kärdla Mälestusmärk: sündmusele; isikule 15. Kalana 25. Sääretirp Tahkuna ps Lehtma 6. Kärdla sadam Skulptuur; arheoloogiline paik Meelste Suurjärv 16. Vanajõe org 26. Kassari kabel ja kabeliaed 7. Ristimägi RMK külastuskeskus; allikas 14 17. Sõru sadam ja muuseum 27. Vaemla villavabrik Kauste 8. Tahkuna tuletorn Austurgrunne Pinnavorm; looduslik huviväärsus 18. Emmaste kirik Meelste laht Park; üksik huvitav puu Hiiu madal 50 Tjuka (Näkimadalad) Suursäär Tõrvanina R Rändrahn; ilus vaade Kodeste ä l Mangu Kersleti b (Kärrslätt) y Metsaonn; lõkkekoht 82 l Malvaste a 50 Ta Tareste mka Kakralaid Borrby h 50 Mudaste res t Karavaniparkla; telkimiskoht te Ogandi o Kootsaare ps Matkarada; vaatetorn Sigala Saxby Tareste KÄRDLA Diby Vissulaid 50 Vormsi Tuulik; tuugen Fällarna Reigi laht Risti 80 välijõusaal Rälby Reigi Roograhu H Kroogi Hausma 50 Tuletorn; mobiilimast Ninalaid Posti Rootsi Kidaste Huitberg 50 Muuseum; kaitserajatis Ninaots a Kanapeeksi Heilu Förby 13 Linnumäe Suuremõisa -

Woodworking in Estonia
WOODWORKING IN ESTONIA HISTORICAL SURVEY By Ants Viires Translated from Estonian by Mart Aru Published by Lost Art Press LLC in 2016 26 Greenbriar Ave., Fort Mitchell, KY 41017, USA Web: http://lostartpress.com Title: Woodworking in Estonia: Historical Survey Author: Ants Viires (1918-2015) Translator: Mart Aru Publisher: Christopher Schwarz Editor: Peter Follansbee Copy Editor: Megan Fitzpatrick Designer: Meghan Bates Index: Suzanne Ellison Distribution: John Hoffman Text and images are copyright © 2016 by Ants Viires (and his estate) ISBN: 978-0-9906230-9-0 First printing of this translated edition. ALL RIGHTS RESERVED No part of this book may be reproduced in any form or by any electronic or mechanical means including information storage and retrieval systems without permission in writing from the publisher, except by a reviewer, who may quote brief passages in a review. This book was printed and bound in the United States. CONTENTS Introduction to the English Language Edition vii The Twisting Translation Tale ix Foreword to the Second Edition 1 INTRODUCTION 1. Literature, Materials & Methods 2 2. The Role Played by Woodwork in the Peasants’ Life 5 WOODWORK TECHNOLOGY 1. Timber 10 2. The Principal Tools 19 3. Processing Logs. Hollowing Work and Sealed Containers 81 4. Board Containers 96 5. Objects Made by Bending 127 6. Other Bending Work. Building Vehicles 148 7. The Production of Shingles and Other Small Objects 175 8. Turnery 186 9. Furniture Making and Other Carpentry Work 201 DIVISION OF LABOR IN THE VILLAGE 1. The Village Craftsman 215 2. Home Industry 234 FINAL CONCLUSIONS 283 Index 287 INTRODUCTION TO THE ENGLISH-LANGUAGE EDITION feel like Captain Pike. -

Seaweed Resources of the Baltic Sea, Kattegat and German and Danish
Botanica Marina 2020; 63(1): 61–72 Review Florian Weinberger*, Tiina Paalme and Sofia A. Wikström Seaweed resources of the Baltic Sea, Kattegat and German and Danish North Sea coasts https://doi.org/10.1515/bot-2019-0019 Received 30 March, 2019; accepted 8 October, 2019; online first 12 Introduction November, 2019 This publication provides an update to an earlier article by Abstract: Due to low salinity and lack of hard substrata, Schramm (1998), who already gave a detailed description the Baltic Sea and Kattegat area and German and Dan- of the macroalgal species distribution and diversity along ish North Sea coasts are characterized by a relatively SE North Sea and Baltic Sea coasts. During the last two low diversity of seaweeds. At the same time the areas decades several species introductions into the region have are severely eutrophicated, which has caused extensive been recorded [for example, approximately 10 on German shifts in macroalgal communities toward opportunistic coasts (Lackschewitz et al. 2014, Steinhagen et al. 2018)] species. Unattached seaweed communities dominated and also range shifts of species were observed within the by Furcellaria lumbricalis, which have been a resource area (Kovtun et al. 2009, Steinhagen et al. 2018). None- for hydrocolloid production since the 1940s, have been theless, the general distribution patterns outlined by severely reduced due to eutrophication and unsustain- Schramm (1998) still remain valid. At the German and able harvesting and are nowadays only exploited com- Danish West coasts, natural hard substratum that would mercially in Estonia. On the other hand, the biomass of allow for algal settlement is extremely rare and is almost opportunistic seaweeds of various red, green and brown only available around the German island of Helgoland, algal genera has increased. -

Snps Reveal Geographical Population Structure of Corallina Officinalis (Corallinaceae, Rhodophyta)
SNPs reveal geographical population structure of Corallina officinalis (Corallinaceae, Rhodophyta) Chris Yesson1, Amy Jackson2, Steve Russell2, Christopher J. Williamson2,3 and Juliet Brodie2 1 Institute of Zoology, Zoological Society of London, London, UK 2 Natural History Museum, Department of Life Sciences, London, UK 3 Schools of Biological and Geographical Sciences, University of Bristol, Bristol, UK CONTACT: Chris Yesson. Email: [email protected] 1 Abstract We present the first population genetics study of the calcifying coralline alga and ecosystem engineer Corallina officinalis. Eleven novel SNP markers were developed and tested using Kompetitive Allele Specific PCR (KASP) genotyping to assess the population structure based on five sites around the NE Atlantic (Iceland, three UK sites and Spain), spanning a wide latitudinal range of the species’ distribution. We examined population genetic patterns over the region using discriminate analysis of principal components (DAPC). All populations showed significant genetic differentiation, with a marginally insignificant pattern of isolation by distance (IBD) identified. The Icelandic population was most isolated, but still had genotypes in common with the population in Spain. The SNP markers presented here provide useful tools to assess the population connectivity of C. officinalis. This study is amongst the first to use SNPs on macroalgae and represents a significant step towards understanding the population structure of a widespread, habitat forming coralline alga in the NE Atlantic. KEYWORDS Marine red alga; Population genetics; Calcifying macroalga; Corallinales; SNPs; Corallina 2 Introduction Corallina officinalis is a calcified geniculate (i.e. articulated) coralline alga that is wide- spread on rocky shores in the North Atlantic (Guiry & Guiry, 2017; Brodie et al., 2013; Williamson et al., 2016). -

Kärdla, Mida Vahel Kutsutakse Hellitavalt Ka Pealin- M [email protected] Naks
Hiiumaa saar koos laidudega kuulub UNESCO biosfäärialade nimestikku. Kestva arengu eelkäijana, keskendub programm Man and Biosphere bio- E loogilise mitmekesisuse ja inimkultuuri tasakaalustatud koosarengusse elukeskkonna säästva majandamise põhimõtteid järgides. Meie väärtus- tame oma unikaalset loodust ja loodame, et ka saare külastajad käituvad vastutustundlikult. & www.hiiumaa.ee Saare keskuseks on Kärdla, mida vahel kutsutakse hellitavalt ka pealin- M [email protected] naks. Juba 14. sajandil paiknes siin väike rootslaste küla, mis hiljem arenes £ (+372) 504 5393 tööstuskeskuseks. Omaaegsetest asukatest annavad märku rootsi päritolu P Hiiu 1 (Keskväljakul), HIIUMAA koha- ning perekonnanimed. Oleme kaardile märkinud ca 5,3 km pikkuse tuuri Kärdla vaatamis- 92413, Kärdla, Hiiumaa TURISMIKAART väärsustest, mille läbimine jalutades võtab aega ca 1-1,5 h. #VisitHiiumaa Legend Kaitseala piir 1 Kärdla tuuri marsruut ja objektid TRANSPORDIINFO Turismiinfokeskus; inva-tualett; tualett Rattamarsruudid: Arstiabi; apteek; politsei Eurovelo marsruut 1 Kärdla KÄRDLA sadam Sadam; slipikoht; navigatsioonimärk marsruut 304 KORK marsruut 305 Kuur Vallavalitsus; bussijaam; raamatukogu 5 Sadama ait Kool; lasteaed; mänguväljak Titekivi S a Tareste laht d Spordirajatis; seikluspark; matkarada Kärdla rand a m 6 Lu b Kultuurimaja; teater; muuseum Rannapaargu ja a Hausma supluskoht rannavõrkpalli- ah väljakud ju Hausma tee Kirik; laululava; muu huvitav hoone N Ööbiku- p õi u park k Mälestusmärk; rändrahn; rand u Rannapark Väike-Sadama L t välijõusaal